Clinical Aspects of BRCA-Associated Breast and Ovarian Cancer
From a clinical perspective, it is important to determine whether or not BRCA-associated malignancies are distinct, either in appearance, biology or outcome. BRCA-associated breast cancers are usually infiltrating ductal carcinomas (NOS), with some series reporting a modest increase in medullary
These tumors are usually poorly-differentiated, although there may be subtle differences in appearance between BRCA1-and BRCA2-associated cancers. Difficulties in obtaining concordance among pathologists with respect to the components of histologic grade preclude using these differences to judge the probability of detecting a mutation in either BRCA1 or BRCA2.
Mucinous histologies and low-grade tumors are under-represented among BRCA-associated ovarian cancers. From a biologic standpoint, BRCA-associated breast cancers tend to be aneuploid with high proliferative rates (by mitotis counts, flow cytometry or Ki-67 staining). The proportion of BRCA1-associated breast tumors that are hormone-receptor negative appears to be greater than expected, even accounting for the young age at onset of these cancers.
The distribution of hormone-receptor status in BRCA2-associated cancers is less clearly defined, but may be more similar to that of nonhereditary cases of comparable age. Although few data are available regarding other biologic parameters, P53 overexpression (and somatic mutation) appears to be common in BRCA-associated breast and ovarian cancer. Interestingly, HER2/neu overexpression appears to be quite uncommon in BRCA-associated breast cancers.
The prognosis of BRCA-associated cancers is a matter of some controversy (reviewed in ref. 21). Most published series have failed to demonstrate inferior survival for breast cancer patients with germline BRCA1 mutations when compared to population controls or women without mutations from the same ascertainment (e.g., early-onset breast cancer). Two series, however, one from Canada and one from France, have suggested that the presence of a BRCA1 mutation may be an adverse prognostic factor for survival.
Most of the published series suffer from a systematic survival bias in that only living women were tested. This bias could obscure an adverse effect of BRCA mutations on survival. In addition, other prognostic features (e.g., nodal status, hormone-receptor status, age) are not routinely taken into account in any of these series.
It is important to note that few data are available specifically regarding the impact of BRCA2 mutations on outcome, and the impact of the two genes may not necessarily be the same. To date, then, there is no conclusive evidence that women with BRCA-associated breast cancer have an inferior outcome when compared to women without mutations with a similar distribution of traditional prognostic factors. The matter requires further study, however, preferably in large groups of unselected, incident cases. As regards ovarian cancer, an early report suggested that women with BRCA-associated ovarian cancer had a superior survival when compared to controls.
Although other small series failed to confirm this observation, a large incident series demonstrating a significantly improved survival for BRCA-associated ovarian cancer has recently been reported. The biologic reason for the improvement in survival remains to be elucidated, and at this time there are no data to indicate that BRCA heterozygotes with ovarian cancer should receive less intensive treatment than women without mutations.
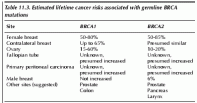
Table 11.3. Estimated lifetime cancer risks associated with germline BRCA mutations and atypical medullary types.
Based on the above data, there is presently no indication that systemic therapy of either breast or ovarian cancer should be modified on the basis of germline BRCA status. Concern has been raised, however, that BRCA heterozygotes with breast cancer might not be appropriate candidates for breast-conservation therapy. While there are few series specifically addressing this issue, 37 heterozygotes at our institution have undergone breast-conservation therapy for 41 breast cancers. With a median follow-up of 77 months for surviving patients, the projected 5 year ipsilateral failure rate is 11.9%.
While this rate of ipsilateral recurrence is comparable to that reported in other series of young women undergoing breast-conservation therapy, it will be extremely important to verify these data on an unselected series of women with mutations.
Until such information is available, current data do not support denying BRCA heterozygotes the opportunity to undergo breast-conservation therapy because of fears about a prohibitive rate of local recurrence. The contralateral breast cancer risk, however, is significant in all women with BRCA1 or BRCA2 mutations, and those women who would consider risk-reducing mastectomy need to be made aware of the impact that radiation therapy may have upon options for reconstruction.
Whether radiation therapy influences the contralateral breast cancer risk in these women is presently unknown.
Mark E. Robson
Breast conservation therapy for invasive breast cancer in Ashkenazi women with BRCA gene founder mutations. J Natl Cancer Inst 2003