Gene Therapy for Breast Cancer
Bone Marrow Protection by Drug-Resistance Gene
Breast cancer is sensitive to chemotherapy. Response rates of advanced breast cancer for most combination chemotherapy are between 40% and 70% (complete response (CR) rate 10-30%), but duration of response is 7-10 months for PR, and 9-18 months for CR. High dose chemotherapy with autologous blood stem cell transplantation for advanced breast cancer has shown high complete response rates (up to 50%), and 10-15% patients have enjoyed durable remission23, 24). However, most patients will relapse after transplantation.
Randomized studies comparing high dose chemotherapy and conventional chemotherapy showed that median survival times appear to be no better than those achieved with conventional chemotherapy, so far25). Probably high dose chemotherapy cannot completely eradicate residual disease, and insufficient bone marrow function after the reconstitution is a major problem in post-transplantation chemotherapy. One approach to overcome the current situation would be the transplantation of the drug-resistant gene-transduced hematopoietic stem cells so that normal bone-marrow cells will be protected from the toxic effect of anticancer drugs.
A multidrug resistance 1 (MDR1) gene was cloned from cancer cell lines resistant to various anticancer drugs26). The MDR1 gene product (P- glycoprotein, P-gp) is a 170 kD glycoprotein consisting of two trans-membranous domains and two ATP-binding domains. P-gp ATP-dependently excretes various drugs such as doxorubicin, vinka-alkaloids, or taxanes from cytoplasm to extra-cellular fluid. Ex vivo transfer of MDR1 genes into hematopoietic stem cells and transplantation might make post-transplant chemotherapy feasible.
Chemotherapeutic drugs such as docetaxel and paclitaxel, which have good clinical activity in the treatment of breast cancer and are efficiently effluxed by P-gp, might be the best choice for this strategy. Using a retroviral vector, Sorrentino et al.27) transplanted MDR1-transduced bone marrow into irradiated mice and then treated them with paclitaxel. Paclitaxel treatment increased MDR1- transduced leukocytes in peripheral blood (in vivo amplification), and MDR1-transduced mice showed reduced bone marrow suppression by paclitaxel (bone marrow protection). Then, several groups have undertaken clinical studies of MDR1 gene therapy for advanced breast cancer or other neoplasms28-30).
A group at MD Anderson Cancer Center first reported the results of clinical trials28). They performed retroviral gene transfer without using cytokines, and in suspension or with autologous stromal cells. In vitro transduction efficiency was 2.8% with the solution method and 5.6% with the stromal method, detected by in situ PCR. But three to four weeks after transplantation, direct PCR assay of peripheral blood leukocytes in patients showed positive results in 0/10 with the solution method, and 5/8 with the stromal method.
These data show insufficient transduction efficiency without using cytokines. NCI also reported the results of a clinical trial of retroviral MDR1 gene therapy30). They transferred MDR1 genes into bone marrow mononuclear cells or peripheral blood stem cells stimulated by IL-3, IL-6, and SCF. Ex vivo transduction efficiency was 0.2-0.5%.
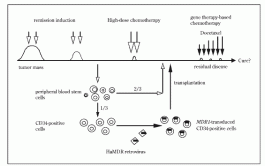 Fig 1. Schema of MDR1 gene therapy for advanced breast cancer patients in Cancer Institute Hospital. They treated transplanted patients with paclitaxel, but they could not show any enrichment of MDR1- transduced white blood cells by PCR. A group at Columbia University also transferred MDR1 genes into bone marrow mononuclear cells or peripheral blood stem cells stimulated by IL-3, IL-6, and stem cell factor (SCF). They showed that 20-70% of BFU- E or CFU-GM colonies from transferred CD34- positive cells were positive for MDR1 by PCR. BM from patients 3-12 weeks after transplantation showed MDR1-positivity by PCR in 2/5 patients.
Fig 1. Schema of MDR1 gene therapy for advanced breast cancer patients in Cancer Institute Hospital. They treated transplanted patients with paclitaxel, but they could not show any enrichment of MDR1- transduced white blood cells by PCR. A group at Columbia University also transferred MDR1 genes into bone marrow mononuclear cells or peripheral blood stem cells stimulated by IL-3, IL-6, and stem cell factor (SCF). They showed that 20-70% of BFU- E or CFU-GM colonies from transferred CD34- positive cells were positive for MDR1 by PCR. BM from patients 3-12 weeks after transplantation showed MDR1-positivity by PCR in 2/5 patients.
They also analyzed P-gp expression in bone marrow cells using flow cytometry, but they could not show any expression. Clinical studies of MDR1 gene therapy are now ongoing at several institutions (Stewart, Cowan, Disseroth, Hesdorffer, O’Shaughnessy).