Assessment of Risk and Choice of Adjuvant Therapy
In order to decide which patients will benefit from the addition of adjuvant therapy, it is important to assess each woman’s risk of developing recurrent disease. Competing comorbid conditions must also be considered. The overview analysis then provide an estimate of the reductions in the risk of relapse that can be expected from the administration of the drug therapies.
With this information, the absolute benefits of therapy can be estimated for each patient. These benefits must be balanced by consideration of the toxicities of the treatment, as well as of the expense and relative inconvenience of the drug administration.
By far the most important predictor for estimating the risk of recurrence is the number of ipsilateral axillary lymph nodes involved with metastatic carcinoma. A patient with even one positive lymph node has a risk of relapse of approximately 30%. An adjuvant therapy that reduces the odds of recurrence by at least 30% at 10 years would improve a woman’s chances of disease-free survival by approximately 9%. Therefore, adjuvant therapy should be strongly considered for these patients.
For patients with negative lymph nodes, the risk of recurrence is lower, and the benefits of therapy must be weighed carefully against the side effects of treatment. Approximately one-quarter of such patients will eventually relapse with recurrent metastatic disease. This supports the concept that local control with surgery and radiation does not prevent the early dissemination of distant metastatic disease. Although all patients are not destined to relapse, it is difficult to select definitively which patients will recur, thereby sparing other patients the unnecessary treatment.
The ability to predict the biological behavior of breast tumors has been an active area of investigation, and potential prognostic factors, including hormone receptor status, S-phase fraction, DNA ploidy and cathepsin D, may help to select a group of patients who would benefit from adjuvant therapy. These variables often correlate with already proven factors such as tumor size and histopathologic features such as degree of differentiation and lymphovascular invasion. For node-negative patients, tumors measuring less than or equal to 1 cm have an excellent prognosis with a risk of relapse of only 12% at 20 years. The risk of relapse and mortality, however, increases with greater tumor size and axillary lymph node involvement (Table 8.3).
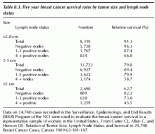 Table 8.3. Five year breast cancer survival rates by tumor size and lymph node status
Table 8.3. Five year breast cancer survival rates by tumor size and lymph node status
Data on 24,740 cases recorded in the Surveillance, Epidemiology, and End Results (SEER) Program of the NCI were used to evaluate the breast cancer survival in a representative sample of women in the United States. From Carter CL, Allen C, and Henson DE. Relation of Tumor Size, Lymph Node Status, and Survival in 24,740 Breast Cancer Cases, Cancer 1989;63:181-187.
Tumors with favorable histologies (including tubular, mucinous, papillary and colloid) have a better outcome, with a low risk of recurrence for tumors up to 3 cm in diameter.
Recently, attention has focused on specific molecular genetic changes as indicators of prognosis. Overexpression/activation of genes that promote cellular transformation, tumor growth and tumor dissemination has been detected in a significant proportion of breast tumors. HER-2/neu is a growth factor receptor that is overexpressed in approximately 20-30% of breast cancers, and HER-2/neu overexpression may predict for a more aggressive tumor biology and natural history of disease. Retrospective analyses of prospective trials have recently suggested that tumors that overexpress the HER-2/neu gene may be more responsive to anthracycline-containing chemotherapy regimens.
Therefore, molecular genetic changes may play an increasingly larger role in the future selection of adjuvant therapy for patients with lymph node-negative disease.
Maura N. Dickler
American Cancer Society Guidelines for the Early Detection of Breast Cancer: Update 2003. CA Cancer J Clin 2003
References
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomized trials. Lancet 1998; 351:1451-1467.
- Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: An overview of the randomized trials. Lancet 1998; 352:930-942.
- Early Breast Cancer Trialists' Collaborative Group. Ovarian ablation in early breast cancer: Overview of the randomized trials. Lancet 1996; 348:1189-1196.
- Fisher B, Costantino JP, Wickerham DL et al. Tamoxifen for the prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst, 1998; 90:1371-1388.
- Gianni Bonadonna, Guest Editor. Breast Cancer. Seminars in Oncology 1996; 23:413-532.