Tamoxifen for Adjuvant Therapy
The largest group of women in the 1995 analysis included almost 37,000 women randomized in 55 trials, all of which were designed to look at whether tamoxifen was an active agent in the postoperative setting. Women who were randomized to tamoxifen were found to have a highly significant reduction in the annual risks of both recurrence and death compared with women who received no adjuvant tamoxifen. This was seen for women who were randomized to tamoxifen for a median duration of 1, 2 or 5 years.
This effect was most pronounced for women who received 5 years of adjuvant tamoxifen, with a reduction in the risk of recurrence of 42% (standard deviation [SD]) and a reduction in the risk of death of 22% (SD 4). A longer duration of tamoxifen is currently being explored; however, at the present time there are no data to suggest an advantage to more than 5 years of tamoxifen.
The dose of tamoxifen that was administered in most of these trials was 20-40 mg each day, and there was no evidence of a dose-response relationship within this range.
Most of the benefit of tamoxifen was noted for women with estrogen-receptor (ER)-positive tumors rather than for patients with ER-poor tumors (Table 8.1). After 5 years of tamoxifen, women with ER-positive tumors had a reduction in the risk of recurrence of 50% (SD 4), compared with a risk reduction of only 6% (SD 11) for women with ER-poor tumors.
Within this “receptor-poor” category, some of the receptors must have been low receptor-positive, which partially accounts for the small positive effect.
The benefits of tamoxifen were most significant in women with the highest estrogen-receptor expression (>100 fmol receptor per mg cytosol protein).
The 1995 analysis was the first overview to provide convincing evidence of a lack of a substantial benefit for the effect of tamoxifen in the ER-poor population, and the hormone-receptor measurement is considered an important determinant of the response to treatment. Although the number of women was small, patients with ER-negative and progesterone-receptor (PR)-positive tumors appeared to benefit from tamoxifen; therefore, this subgroup most likely represents a tamoxifen-responsive population.
The proportional benefits of tamoxifen are similar for women with both lymph node-positive and node-negative disease. The absolute benefits, however, from the use of tamoxifen are related to each woman’s risk of recurrence. Women with lymph node-positive disease have a higher annual risk of recurrence and mortality from breast cancer and therefore derive a greater absolute benefit from the use of tamoxifen (Fig. 8.1).
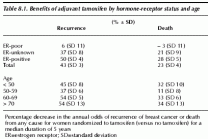 Table 8.1. Benefits of adjuvant tamoxifen by hormone-receptor status and age
Table 8.1. Benefits of adjuvant tamoxifen by hormone-receptor status and age
The effects of tamoxifen are apparent on the risks of recurrence after the first year of treatment, with most of the benefits seen during the initial 5 years of therapy. The effects of tamoxifen on mortality, however, continue to increase even after the completion of 5 years of therapy, with a significant difference noted at 10 years.
In previous overviews, the benefits of tamoxifen appeared limited to postmenopausal women (defined as age >50); however, with longer follow-up and a greater number of premenopausal women included in the analysis, the 1995 overview has extended the benefits of tamoxifen to women less than and greater than 50 years of age (Table 8.1). Therefore, age and menopausal status should no longer represent a barrier to the use of tamoxifen, and the majority of women with ER-positive tumors should be offered tamoxifen as part of adjuvant therapy.
Although tamoxifen was shown to reduce the risk of recurrence and mortality in women with breast cancer, tamoxifen was associated with an increased incidence of endometrial carcinoma. The risk of invasive endometrial cancer was increased about 2.5-fold among tamoxifen-treated women, with a higher risk for women who received 5 years of therapy (RR 4.2, 2-sided p <0.0001). Other trials have also demonstrated an increased risk of endometrial cancer with the use of tamoxifen.
 Fig. 8.1. Absolute risk reductions for 5 years of adjuvant tamoxifen during the first 10 years, subdivided by nodal status (after exclusion of women with ER-poor disease). In these generalized Kaplan-Meier curves, the values for the tamoxifen and control patients at 5 years and at 10 years are given beside each pair of lines. Differences in 10 year outcome, together with their standard errors, are given below. 1998 Lancet.
Fig. 8.1. Absolute risk reductions for 5 years of adjuvant tamoxifen during the first 10 years, subdivided by nodal status (after exclusion of women with ER-poor disease). In these generalized Kaplan-Meier curves, the values for the tamoxifen and control patients at 5 years and at 10 years are given beside each pair of lines. Differences in 10 year outcome, together with their standard errors, are given below. 1998 Lancet.
In NSABP-B-14, a randomized adjuvant trial of tamoxifen, 23 cases of endometrial cancer were reported in women receiving tamoxifen compared with two cases in the control group (both of whom had subsequently required tamoxifen due to a change in medical status). This translated into a relative risk of 7.5 (95%, CI 1.7-32.7) for the tamoxifen-treated patients, and a 5 year cumulative hazard rate of 6.3 per 1,000. The vast majority of cases (21 of 24) were International Federation of Gynecology and Obstetrics (FIGO) Stage I, with most cases of good to moderate histologic grade.
Tamoxifen is considered the first choice of adjuvant therapy for post-menopausal women with ER-positive tumors as it is well tolerated with minimal toxicity. Tamoxifen also adds to the benefits of chemotherapy in all women with receptor-positive disease. The combination of chemotherapy with tamoxifen will be discussed further below. Other hormonal therapies, including the aromatase inhibitor anastrozole, are presently being compared to tamoxifen in adjuvant clinical trials.
Maura N. Dickler
American Cancer Society Guidelines for the Early Detection of Breast Cancer: Update 2003. CA Cancer J Clin 2003
References
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomized trials. Lancet 1998; 351:1451-1467.
- Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: An overview of the randomized trials. Lancet 1998; 352:930-942.
- Early Breast Cancer Trialists' Collaborative Group. Ovarian ablation in early breast cancer: Overview of the randomized trials. Lancet 1996; 348:1189-1196.
- Fisher B, Costantino JP, Wickerham DL et al. Tamoxifen for the prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst, 1998; 90:1371-1388.
- Gianni Bonadonna, Guest Editor. Breast Cancer. Seminars in Oncology 1996; 23:413-532.