Dilemma of “Earlier” detection
However, one might extrapolate from certain experiences to suggest that among women at a given point in the continuum, not all will progress to the next or subsequent steps. For example, a number of pathological features such as the presence of axillary lymph nodal micrometastases are known to identify women more likely to develop morbidity and mortality after local-regional therapy. However, 20 to 30 percent of node-negative patients subsequently develop symptomatic, incurable metastases, and up to 25 percent of node-positive patients do not (Cocconi, 1995; Weidner, 1995). More recently, clinical investigators have reported that detection of distant micrometastatic breast cancer cells in circulation or even bone marrow is associated with a poorer prognosis (Braun et al., 2000; Pantel et al., 1999). However, a substantial fraction of these “positive” patients also survive without systemic therapy. Other molecular features, such as hormone receptor content, measures of proliferation, and HER-2 gene amplification, may help to fine-tune prognostic estimates, but they are far from absolute in dividing a population into those who are destined to develop “disease” and those who are not.
DCIS presents an even greater dilemma than early invasive cancers.
DCIS hypothetically lies to the left of invasive cancer on the theoretical continuum illustrated in Figure 1-6. Since DCIS does not contain cells that have invaded the surrounding tissue layer, it should not be associated with metastatic cells or a very high, if any, risk of developing morbidity and mortality. Therefore, in theory, DCIS would be the preferred lesion to detect by screening (rather than invasive cancer). However, it is not known what percentage of breast cancers pass through the DCIS phase.
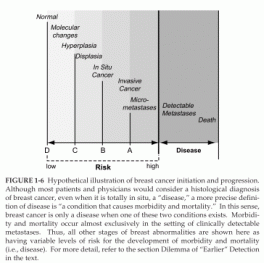
FIGURE 1-6 Hypothetical illustration of breast cancer initiation and progression.
In other words, do some breast cancers progress from left to right in Figure 1-6 with only a brief sojourn as DCIS, or do some perhaps skip it completely? Moreover, few patients who have been diagnosed with this condition have ever been studied or followed without excising of the lesion. Therefore, it is not known whether some or many in situ breast cancers would never progress beyond this phase. Indeed, it is likely that a certain proportion of these lesions might remain as in situ cancer or even regress to a less worrisome histologic state. Nonetheless, recent randomized clinical trials have suggested that women with excised DCIS who do not receive further local (surgery, radiation) or systemic (hormonal) therapy are more likely to suffer a recurrence of cancer in the breast with either the same diagnosis or a more advanced diagnosis (further to the right on the continuum illustrated in Figure 1-6), such as invasive breast cancer (Fisher et al., 1998; Hetelekidis et al., 1999; Silverstein and Lagios, 1997).
In summary, current definitions of breast cancer, as determined by histopathology of biopsy specimens taken from the breast, are only imperfect surrogates of whether patients will develop true disease, as defined here. A diagnosis of anything other than “normal” or “benign” breast tissue places the patient into a higher risk category, whereas treatment with surgery or with radiation or systemic therapy places the patient in a lower risk category. Treatment in this setting is always applied inefficiently because there is no effective way of determining who will benefit from it. Thus, many women receive “preventive” therapy, even though they would never develop metastatic disease, in order to reduce the risk of morbidity and mortality for those who would. Preventive therapy is less efficient the further to the left on the breast cancer continuum in Figure 1-6 one goes. As a woman’s condition moves farther to the right on the continuum, women are more willing to accept previously unacceptable preventive therapies because they perceive their risk of death from breast cancer to be higher.
###
Sharyl J. Nass, I. Craig Henderson, and Joyce C. Lashof
Committee on Technologies for the Early Detection of Breast Cancer
National Cancer Policy Board INSTITUTE OF MEDICINE and Division of Earth and Life Studies
NATIONAL RESEARCH COUNCIL