Dilemma of “Earlier” detection
The efforts to develop technologies capable of pushing back the detection timeline to a stage that is even earlier than what is currently possible could provide new opportunities for early intervention, but such opportunities could also increase the difficulties associated with “overdiagnosis.” The underlying assumption in promoting early detection held by many investigators, most physicians, and almost all members of the lay population is that early detection provides an opportunity to reverse the malignancy process more effectively and perhaps with less toxicity than if the condition were identified later.
The notion that earlier detection is better requires the following three assumptions: (1) that development of carcinoma of the breast proceeds through a relatively orderly series of steps (Figure 1-6); (2) that this process includes an irreversible checkpoint (which may vary for different patients) and that once an individual is past this checkpoint the process steadily progresses so that only some sort of external intervention can prevent the ultimate inevitability of morbidity and mortality; and (3) that detection of this process before establishment of metastatic deposits will permit external intervention to prevent disease-specific morbidity and mortality more efficiently than after metastases are established. These points of detection are illustrated as A, B, C, and D, respectively by the vertical lines on Figure 1-6.
The fundamental support for these assumptions comes from the randomized clinical trials described earlier in this section, which showed that mammography screening of asymptomatic women results in early treatment of breast cancers that are detected and reduces disease-specific mortality by approximately 20 to 30 percent compared with that for women who have not been screened. Why is the decrease in mortality not greater? There are several possible answers to this question. Simply put, screening mammography is insufficiently sensitive in two ways: (1) it does not detect all cancers at point B, and (2) it does not detect any cancers at point C.
One might argue that improvements in imaging technologies might allow clinicians to increase the number of tumors detected at point B or even move detection to point C. However, a second reason that reductions in breast cancer mortality rates due to screening mammography are limited might be embedded in the biology of the cancer itself. Some cancers may metastasize at a time well before point B on the breast cancer continuum, whereas others may do so much later. Thus, the question of whether detection is early enough to allow effective intervention may vary from one woman to the next.
The assumptions presented above raise several considerations. A distinction should be made between screening for an asymptomatic but precise condition that is considered undesirable and establishing that the individual is at risk for such a condition but does not yet and may never have it. Indeed, taken to its extreme, this distinction is blurred and perhaps even abolished in the definition of “disease.” For example, most patients and physicians would consider a histological diagnosis of breast cancer, even when it is totally in situ, a “disease.” However, a more precise definition of disease is “a condition that causes morbidity and mortality.” In this sense, breast cancer is only a disease when one of these two conditions exists. Morbidity and mortality occur almost exclusively in the setting of clinically detectable metastases.
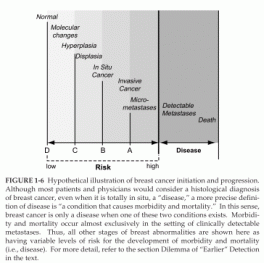
FIGURE 1-6 Hypothetical illustration of breast cancer initiation and progression.
Although most patients and physicians would consider a histological diagnosis of breast cancer, even when it is totally in situ, a “disease,” a more precise definition of disease is “a condition that causes morbidity and mortality.” In this sense, breast cancer is only a disease when one of these two conditions exists. Morbidity and mortality occur almost exclusively in the setting of clinically detectable metastases. Thus, all other stages of breast abnormalities are shown here as having variable levels of risk for the development of morbidity and mortality (i.e., disease). For more detail, refer to the section Dilemma of “Earlier” Detection in the text.
Before the increased awareness of breast cancer and adoption of screening mammography, almost all patients who presented with breast cancer did so because the condition was at a stage at which it was truly a disease: it caused symptoms or even death. However, in more modern times, most patients are diagnosed with asymptomatic breast cancer. In this setting, almost all treatment for breast cancer (surgical removal of the primary tumor, local-regional radiation to sterilize the area, and adjuvant systemic therapy) could be considered prophylactic or preventive. Such treatments are applied to reduce the chances that the patient will develop morbidity or mortality (or “disease”). A substantial body of literature describing the results of randomized clinical trials demonstrates that each of these strategies does, in fact, reduce to some extent the individual’s chances of developing morbidity or mortality from breast cancer.
Why are these distinctions important? No single condition, other than actually having symptomatic disease, carries a 100 percent chance (or risk) of developing what is designated, in this discussion, disease. However, as one moves from left to right on the theoretical breast cancer continuum illustrated in Figure 1-6, an individual’s chances of ultimately developing morbidity and mortality increase. Estimating this risk is quite difficult because, at least in the last century, few patients were left untreated once they had a diagnosis of “breast cancer,” and therefore, it is impossible to determine what percentage of patients, if any, might never have progressed to experience morbidity or mortality from breast cancer if they were left untreated.