Normal Breast Development During Puberty
Puberty in girls begins at the age of 10 to 12 years as a result of the influence of hypothalamic gonadotropin-releasing hormones secreted into the hypothalamic–pituitary portal venous system. The basophilic cells of the anterior pituitary release follicle-stimulating hormone and luteinizing hormone. Follicle-stimulating hormone causes the primordial ovarian follicles to mature into graafian follicles, which secrete estrogens, primarily in the form of 17b-estradiol. These hormones induce the growth and maturation of the breasts and genital organs.
During the first 1 to 2 years after menarche, hypothalamic-adenohypophyseal function is unbalanced, because the maturation of the primordial ovarian follicles does not result in ovulation or a luteal phase. Therefore, ovarian estrogen synthesis predominates over luteal progesterone synthesis. The physiologic effect of estrogens on the maturing breast is to stimulate longitudinal ductal growth of ductal epithelium. Terminal ductules also form buds that precede further breast lobules. Simultaneously, periductal connective tissues increase in volume and elasticity, with enhanced vascularity and fat deposition.
These initial changes are induced by estrogens synthesized in immature ovarian follicles, which are anovulatory; subsequently, mature follicles ovulate, and the corpus luteum releases progesterone. The relative role of these hormones is not clear. In experimental studies, estrogens alone induce a pronounced ductular increase, whereas progesterone alone does not. The two hormones together produce full ductular-lobular-alveolar development of mammary tissues.
The marked individual variation in development of the breast makes it impossible to categorize histologic changes on the basis of age. Breast development by age has been described by external morphologic changes.
Morphology
Adult Breast
The adult breast lies between the second and sixth ribs in the vertical axis and between the sternal edge and the midaxillary line in the horizontal axis (Fig. 1-1). The average breast measures 10 to 12 cm in diameter, and its average thickness centrally is 5 to 7 cm. Breast tissue also projects into the axilla as the axillary tail of Spence. The contour of the breast varies but is usually domelike, with a conical configuration in the nulliparous woman and a pendulous contour in the parous woman. The breast comprises three major structures: skin, subcutaneous tissue, and breast tissue, with the last comprising both parenchyma and stroma. The parenchyma is divided into 15 to 20 segments that converge at the nipple in a radial arrangement. The collecting ducts that drain each segment are 2 mm in diameter, with subareolar lactiferous sinuses of 5 to 8 mm in diameter. Between five and ten major collecting milk ducts open at the nipple.
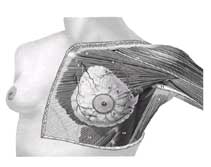
FIG. 1. Normal anatomy of the breast and pectoralis major muscle.
The nomenclature of the duct system is varied. The branching system can be named in a logical fashion, starting with the collecting ducts in the nipple and extending to the ducts that drain each alveolus, as shown in Table 1-2.
- Major ducts
- Collecting ducts
- Lactitrous sinuses
- Segmental ducts
- Subsegmental ducts
- Termnial duct-lobular unit
- Terminal ducts
Extralobular
Intralobular - Lobules
- Alveoli
TABLE 2. Nomenclature of the Breast Epithelial System
Each duct drains a lobe made up of 20 to 40 lobules. Each lobule consists of ten to 100 alveoli or tubulosaccular secretory units. The microanatomy has been described in detail by Parks. The stroma and subcutaneous tissues of the breast contain fat, connective tissue, blood vessels, nerves, and lymphatics.
The skin of the breast is thin and contains hair follicles, sebaceous glands, and eccrine sweat glands. The nipple, which is located over the fourth intercostal space in the nonpendulous breast, contains abundant sensory nerve endings, including Ruffini-like bodies and end bulbs of Krause. Moreover, sebaceous and apocrine sweat glands are present, but not hair follicles. The areola is circular and pigmented, measuring 15 to 60 mm in diameter. The Morgagni tubercles, located near the periphery of the areola, are elevations formed by openings of the ducts of the Montgomery glands. The Montgomery glands are large sebaceous glands capable of secreting milk; they represent an intermediate stage between sweat and mammary glands. Fascial tissues envelop the breast. The superficial pectoral fascia envelops the breast and is continuous with the superficial abdominal fascia of Camper. The undersurface of the breast lies on the deep pectoral fascia, covering the pectoralis major and anterior serratus muscles. Connecting these two fascial layers are fibrous bands (Cooper suspensory ligaments) that represent the “natural” means of support of the breast.
Blood Supply of the Breast
The principal blood supply to the breast is derived from the internal mammary and lateral thoracic arteries. Approximately 60% of the breast, mainly the medial and central parts, is supplied by the anterior perforating branches of the internal mammary artery. Approximately 30% of the breast, mainly the upper, outer quadrant, is supplied by the lateral thoracic artery. The pectoral branch of the thoracoacromial artery; the lateral branches of the third, fourth, and fifth intercostal arteries; and the subscapular and thoracodorsal arteries all make minor contributions to the blood supply.
Lymphatic Drainage of the Breast
Lymph Vessels
The subepithelial or papillary plexus of the lymphatics of the breast is confluent with the subepithelial lymphatics over the surface of the body. These valveless lymphatic vessels communicate with subdermal lymphatic vessels and merge with the Sappey subareolar plexus. The subareolar plexus receives lymphatic vessels from the nipple and areola and communicates by way of vertical lymphatic vessels equivalent to those that connect the subepithelial and subdermal plexus elsewhere. Lymph flows unidirectionally from the superficial to deep plexus and from the subareolar plexus through the lymphatic vessels of the lactiferous duct to the perilobular and deep subcutaneous plexus. The periductal lymphatic vessels lie just outside the myoepithelial layer of the duct wall. Flow from the deep subcutaneous and intramammary lymphatic vessels moves centrifugally toward the axillary and internal mammary lymph nodes. Injection studies with radiolabeled colloid have demonstrated the physiology of lymph flow and have countered the old hypothesis of centripetal flow toward the Sappey subareolar plexus. Approximately 3% of the lymph from the breast is estimated to flow to the internal mammary chain, whereas 97% flows to the axillary nodes. Drainage of lymph to the internal mammary chain may be observed after injection of any quadrant of the breast.
Axillary Lymph Nodes
The topographic anatomy of the axillary lymph nodes has been studied as the major route of regional spread in primary mammary carcinoma. The anatomic arrangement of the axillary lymph nodes has been subject to many different classifications. The most detailed studies are those of Pickren, which show the pathologic anatomy of tumor spread. Axillary lymph nodes can be grouped as the apical or subclavicular nodes, lying medial to the pectoralis minor muscle, and the axillary vein lymph nodes, grouped along the axillary vein from the pectoralis minor muscle to the lateral limit of the axilla; the interpectoral (Rotter) nodes, lying between the pectoralis major and minor muscles along the lateral pectoral nerve; the scapular group, comprising the nodes lying along the subscapular vessels; and the central nodes, lying beneath the lateral border of the pectoralis major muscle and below the pectoralis minor muscle (Fig. 1-2). Other groups can be identified, such as the external mammary nodes lying over the axillary tail and the paramammary nodes located in the subcutaneous fat over the upper, outer quadrant of the breast.
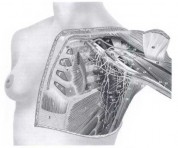 FIG. 2. Chest wall muscles and vascular anatomy.
FIG. 2. Chest wall muscles and vascular anatomy.
An alternative method of delineating metastatic spread, for the purposes of determining pathologic anatomy and metastatic progression, is to divide the axillary lymph nodes into arbitrary levels. Level I lymph nodes lie lateral to the lateral border of the pectoralis minor muscle, level II nodes lie behind the pectoralis minor muscle, and level III nodes are located medial to the medial border of the pectoralis minor muscle (Fig. 1-3). These levels can be determined accurately only by marking them with tags at the time of surgery.
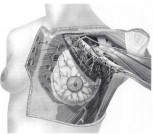 FIG. 3. Lymphatic drainage of the breast showing lymph node groups and levels.
FIG. 3. Lymphatic drainage of the breast showing lymph node groups and levels.
Internal Mammary Lymph Nodes
The internal mammary nodes lie in the intercostal spaces in the parasternal region. The nodes lie close to the internal mammary vessels in extrapleural fat and are distributed in the intercostal spaces, as shown in Fig. 1-3. From the second intercostal space downward, the internal mammary nodes are separated from the pleura by a thin layer of fascia in the same plane as the transverse thoracic muscle. The number of lymph nodes described in the internal mammary chain varies. The nodes lie medial to the internal mammary vessels in the first and second intercostal spaces in 88% and 76% of cases, respectively, whereas they lie lateral to the vessels in the third intercostal space in 79% of cases. The prevalence of nodes in each intercostal space is as follows: first space, 97%; second space, 98%; third space, 82%; fourth space, 9%; fifth space, 12%; and sixth space, 62%. The pathologic anatomy of this route of lymphatic drainage in the spread of breast disease has been described by Handley and Thackray and Urban and Marjani.
In the presence of nodal metastases, obstruction of the physiologic routes of lymphatic flow may occur, and alternative pathways may then become important. The alternative routes that have been described are deep, substernal, cross drainage to the contralateral internal mammary chain superficial presternal crossover, lateral intercostal, and mediastinal drainage and spread through the rectus abdominis muscle sheath to the subdiaphragmatic and subperitoneal plexus (the Gerota pathway). This last route allows the direct spread of tumor to the liver and retroperitoneal lymph nodes. Substernal crossover is demonstrable by isotope imaging of the lymph nodes44 and may be of significance in early breast cancer.
M. P. Osborne: Department of Surgery, Joan and Sanford I. Weill Medical College, Cornell University
New York Presbyterian Hospital, New York, New York