Health Centers > Cancer Health Center > Prostate Cancer
Prostate Cancer
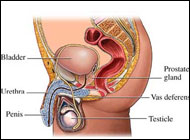
The prostate gland is the male organ most commonly afflicted with either benign or malignant neoplasms. It comprises the most proximal aspect of the urethra. Anatomically it resides in the true pelvis, separated from the pubic symphysis anteriorly by the retropubic space (space of Retzius). The posterior surface of the prostate is separated from the rectal ampulla by Denonvilliers' fascia. The base of the prostate is continuous with the bladder neck, and the apex of the prostate rests on the upper surface of the urogenital diaphragm. Laterally, the prostate is related to the levator ani musculature. Its arterial blood supply is derived from branches of the internal iliac artery (inferior vesical and middle rectal arteries). Venous drainage is via the dorsal venous complex, which receives the deep dorsal vein of the penis and vesical branches before draining into the internal iliac veins. Innervation is from the pelvic plexus. The normal prostate measures 3-4 cm at the base, 4-6 cm in cephalocaudad, and 2-3 cm in anteroposterior dimensions.
McNeal has popularized the concept of zonal anatomy of the prostate. Three distinct zones have been identified (Figure 22-1). The peripheral zone accounts for 70% of the volume of the young adult prostate, the central zone accounts for 25%, and the transition zone accounts for 5%. These anatomic zones have distinct ductal systems but, more important, are differentially afflicted with neoplastic processes. Sixty to 70 percent of carcinomas of the prostate (CaP) originate in the peripheral zone, 10-20% in the transition zone, and 5-10% in the central zone (McNeal et al, 1988). Benign prostatic hyperplasia uniformly originates in the transition zone (Figure 22-2). See Benign prostatic hyperplasia ...
Adenocarcinoma of the prostate gland is the most common noncutaneous neoplasm in men. Every year approximately 200,000 new cases are identified in the United States. Prostate cancer kills about 32,000 men yearly and is second only to lung cancer as a cause of cancer deaths in men. The number of men dying from prostate cancer in the U.S. began to decrease slightly in 1995. The disease is more common and often more aggressive in African-American men. In relation to total deaths from all cancers, prostate cancer is responsible for 6% of cancer deaths in whites and 9% in African Americans. A positive family history of prostate cancer in a first-degree relative is the most important risk factor for prostate cancer. Only about 25% of men who develop clinically recognized prostate cancer die from it; 30% to 40% of men over age 50 have clinically silent prostate cancer that never results in any health consequences. About 40% of deaths from prostate cancer occur in men ages 80 or older.
Prostate Cancer Introduction
The primary care internist is likely to be confronted with an abnormal finding on digital rectal examination of the prostate gland or an elevated prostate-specific antigen value during the routine examination of patients. The frequency of prostate cancer in the general population and the sensitivity of prostate cancer to medical therapy warrant a review of its management for the internist. An estimated 334,500 cases of prostate cancer will be discovered in 1997, and 41,800 deaths are expected in the same year. As a consequence, prostate cancer represents the second most common cause of cancer (behind skin cancer) and the third most common cause of cancer death (behind lung cancer and colorectal cancer) in males in the United States.
Prostate Cancer Relevant Physiology and Pathophysiology
Male gender and age are the most important risk factors for prostate cancer. Although extraordinarily rare in men under the age of 40, prostate cancer reaches a prevalence of 10% in men during the sixth decade of life and nearly 40% by the ninth decade. However, most prostate cancer is occult; only 1% to 2% of men in the ninth decade of life annually manifest clinical evidence of new prostate cancer. Ambient plasma androgen concentrations appear to correlate with the incidence of prostate cancer. Castrated men, men with chronic liver disease who have elevated levels of plasma estrogen, and men with inborn defects of dihydrotestosterone production have lower or negligible risks of prostate cancer. Plasma testosterone concentrations appear to account in part for racial differences in prostate cancer incidence. Black men have the highest incidence of prostate cancer worldwide, American white men have intermediate risk, and Asianmen the lowest risk; these trends in risk correlate with racial differences in inherent androgen concentrations.
The histopathologic precursor for prostate cancer is prostatic intraepithelial neoplasia (PIN) or carcinoma in situ. Benign prostatic hyperplasia (BPH) is not a risk factor for prostatic malignancy. Benign prostatic hyperplasia occurs most commonly within the prostate transition zone, a superficial and relatively small proportion of the gland near the prostatic urethra. 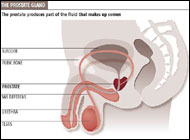 In contrast, PIN and prostate cancer most often arise within the peripheral zone of the gland, the largest component of the gland located near the base of the gland and extending to its apex. More than 95% of prostate cancers are adenocarcinomas originating from the glandular acinae and their proximal ducts; tumors arising from these structures are typically responsive to hormonal therapy. The less common histopathologic types of prostate cancer include small cell cancer, carcinoid tumors, adenoid-cystic cancer, mucinous cancer, and sarcomas, which are not typically sensitive to hormonal therapies.
In contrast, PIN and prostate cancer most often arise within the peripheral zone of the gland, the largest component of the gland located near the base of the gland and extending to its apex. More than 95% of prostate cancers are adenocarcinomas originating from the glandular acinae and their proximal ducts; tumors arising from these structures are typically responsive to hormonal therapy. The less common histopathologic types of prostate cancer include small cell cancer, carcinoid tumors, adenoid-cystic cancer, mucinous cancer, and sarcomas, which are not typically sensitive to hormonal therapies.
The most useful clinical-pathologic system for classifying prostate cancer is the Gleason grading system (Fig. 97-2). The surgical pathologist examining the biopsied or resected prostatic adenocarcinoma evaluates the primary or dominant degree of differentiation of the tumor and the secondary or next most common degree of differentiation of the tumor. A score (1 to 5) is applied to each of these areas of tumor, and the scores are added. The sum of the scores provides the most consistent assessment of prognosis, with a higher score correlating with a greater frequency of mortality. More recent work has sought to associate other biologic characteristics of prostate carcinomas with clinical behavior and prognosis. Excess DNA content (ploidy); abnormalities of chromosomes 7, 8, 10, or 12; and expression of oncogenes or mutated tumor suppressor genes are beginning to yield important leads to the understanding of prostate cancer carcinogenesis, prognosis, and tumor biology.
Prostate Cancer Laboratory and Other Diagnostic Tests
Recent advances have been made in the clinical screening for prostate cancer. Because prostate cancer frequently occurs as an occult condition, may be curable when still localized to the gland, and remains resistant to cure when locally advanced (extending beyond the capsule of the gland) or metastatic to pelvic lymph nodes, distant organs, or bones, there is strong motivation to improve the early detection capability. Annual digital rectal examination (DRE) and serum prostate-specific antigen (PSA) determination remain the chief components of screening recommendations by the American Urological Association and the American Cancer Society. Onset of screening is recommended for all men over the age of 50 years. For black men and men with a family history of prostate cancer, onset of screening is recommended at age 40.
DRE is a simple procedure whose sensitivity for prostate cancer is 86% but whose specificity is only 44%. The rate of detection of prostate cancer by DRE alone in asymptomatic men ranges from 0.2% to 2.2%.
PSA is a serine protease found exclusively in benign and malignant prostate tissue. Elevation of serum PSA may occur in BPH, inflammation or infection of the prostate, or prostate cancer. PSA elevations above 10 ng/ml usually reflect malignant disease of the gland and are rarely observed in individuals with benign conditions. PSA determination with a value of 4 ng/ml as an upper limit of normal provides a 79% sensitivity and a 59% specificity for the detection of prostate cancer. The rate of detection of prostate cancer using PSA determination alone in asymptomatic men is 2.2% to 2.6%. Estimates of PSA concentration in prostatic tissue (PSA density), the rate of rise of PSA values over time (PSA velocity), or the creation of age-specific normal reference ranges for PSA may have greater discriminatory value for cancer detection, particularly for men with PSA values in the troublesome range of 4 to 10 ng/ml.
Transrectal ultrasound (TRUS) is a screening method whereby an endorectal probe housing an ultrasonic transducer is inserted into the rectum overlying the prostate gland. The transducer allows imaging of the prostate for echogenicity, which permits localization of areas suspected for malignancy. Hypoechogenic areas in the peripheral zone of the prostate may be identified that are suspected for malignancy and that may be biopsied for histopathologic confirmation of cancer. As an isolated screening tool, TRUS detects prostate cancer at a rate of 1.7% to 20.6% in screened men. The disadvantages of TRUS include its relatively low sensitivity and specificity for the detection of cancer, its expense, and its requirement for a physician sophisticated in its use. The utility of TRUS derives from its ability to detect nonpalpable cancers, its use as an adjunct to DRE in evaluating palpable lesions, and its ability to enhance the accuracy of biopsy of suspected prostatic lesions.
Serum prostatic acid phosphatase determination was widespread as a screening test before the advent of PSA in clinical practice. The prostatic acid phosphatase value is normal in approximately 57% to 75% of men with localized prostate cancer. Prostatic acid phosphatase testing has been largely supplanted by PSA as the screening test of choice.
The diagnosis of prostate cancer is based on the careful histopathologic examination of prostate resection specimens or specimens collected from prostate biopsy. In the examination of tissue obtained from patients with suspected localized prostate cancer, Gleason scoring should be sought (Fig. 97-2). The probability of extension of cancer beyond the prostatic capsule and into pelvic lymph nodes increases with increasing Gleason score. Once the diagnosis of prostate cancer is confirmed by histopathologic examination, noninvasive evaluation of the patient for metastatic disease should be conducted. The radionuclide bone scan is the most sensitive method for the detection of bone metastases, the most common site of extrapelvic advanced disease in the prostate cancer patient. Plain x-rays of bone in areas showing uptake of radionucliude should be obtained, particularly if in weight-bearing sites or if correlated with pain. Metastasis to visceral or solid organs is relatively uncommon in prostate cancer, whereas metastases to pelvic and abdominal lymph nodes is a frequent route of dissemination. In the 25% of men with metastatic prostate cancer who have pulmonary metastases, routine chest x-ray is a useful procedure. Chest x-ray may not only delineate pulmonary abnormalities but may also detect bone metastases. Computed tomography (CT) and magnetic resonance imaging (MRI) are relatively expensive, low-yield, and insensitive screening procedures for detecting advanced prostate cancer at these sites. PSA determination may be used in the therapeutic management of prostate cancer. Failure of an elevated preoperative PSA value to decline to normal following prostate resection is strong indirect evidence of incomplete resection or disseminated disease. Serial PSA determinations allow the clinician to assess the progress of the patient with disseminated disease receiving systemic therapy.
Screening for Prostate Cancer
The American Cancer Society and the American Urological Association recommend screening with an annual digital rectal exam beginning at age 40. These groups also recommend an annual serum PSA level beginning at age 40 to 45 for African-American men or men who have a family history of prostate cancer, and for all men starting at age 50. The American Academy of Family Physicians and American College of Physicians recommend the physician discuss the risks and benefits of screening and decide based on individual patient preference. In 1996, the U.S. Preventative Services Task Force recommended against screening and it is likely their position will not change until 2004, when evidence from randomized controlled trials on the benefit of screening becomes available.
The presence of a hard nodule, significant asymmetry, or an area of induration on DRE is suspicious for cancer. PSA is an enzyme produced by epithelial cells of the prostate that hydrolyzes the ejaculate and has a function in male fertility. The PSA level can be elevated in BPH, prostatitis, prostate cancer, prostate trauma, prostate surgery, and after an ejaculation. "Routine" DRE does not raise the PSA significantly. The normal PSA level is less than 4 ng/mL. Approximately 20% of men with prostate cancer have a PSA level of 2 to 4. Prostate cancer is rare (<1%) if the PSA is less than 2. Reducing the lower limit of PSA to 2.5 ng/mL would to improve the sensitivity of the test. The improvement in sensitivity would be offset by a large increase in false-positive test results requiring additional diagnostic tests and producing substantial patient anxiety. Levels between 4 and 10 ng/mL may be the result of benign or malignant disease, but only 25% of those in this range will have cancer. As the level rises above 10 ng/mL the probability of cancer increases to 50% or greater. A PSA value above 50 ng/mL is highly suggestive of metastatic disease. In addition to the absolute level of PSA, age-specific normal levels, the rate of increase of PSA, and the ratio of free PSA to total PSA have been used to improve the predictive value; however, there is no conclusive evidence to suggest that these techniques be used routinely. A PSA level that increases by 0.75 ng/mL/year or more may indicate a developing malignancy. If patients are being treated with finasteride, the normal value for PSA should be reduced by 50%.
Cancers detected by PSA screening are equivalent in malignant grade to those diagnosed clinically. Radical prostatectomy or radiation therapy are options for treating cancer that is confined to the prostate, but more than 10 years is required before an increase in survival is evident. The argument for screening is based on the concept that surgery or radiotherapy produces a survival benefit for confined asymptomatic cancer that is likely to become clinically significant before the patient dies from another cause. This situation is most likely in young patients who harbor silent high-grade tumors. It is difficult to justify screening a man who is not expected to live at least 10 years. For healthy men under age 70 and extremely healthy and vigorous men over age 70, individual factors should be weighed in the decision to recommend screening. African Americans and men with a family history of prostate cancer are at greater risk for cancer, and screening these groups should confer a larger benefit. The patient must be informed that abnormal findings detected will lead to urologic consultation, biopsy, and the possibility of surgery. For patients who are offered screening, the DRE and PSA are complementary and should both be performed. Significant abnormalities of DRE or PSA should be referred to a urologist for further evaluation. Until 2004, when the results of randomized prospective trials become available, controversy about the benefit of prostate cancer screening will continue.
Prostate Cancer Diagnosis and Staging
Tests to diagnose prostate cancer
These tests are most commonly used to diagnose prostate cancer:
Digital rectal exam (DRE): The most common and simplest screening test for prostate cancer is the digital rectal exam (DRE). The doctor will gently insert a gloved finger into the rectum to determine if the prostate is enlarged or has lumps. This is not a definitive test, but regular exams can help detect changes in the prostate over time. A DRE may also be used to tell if cancer has spread or returned after treatment.
Prostate-specific antigen (PSA) test: A test that measures the level of PSA, a substance made by the prostate, in the blood. Higher levels of PSA in the blood may indicate prostate cancer. A PSA test is a helpful screening tool for doctors, as it determines the next step in evaluating a patient. If prostate cancer is diagnosed, PSA levels are then most helpful in planning treatment, judging treatment effectiveness, and monitoring the prostate for growth (also called active surveillance or watchful waiting).
It is important to note that PSA levels can fluctuate and are not always a marker for prostate cancer. For example, high PSA levels may be a result of infection, inflammation, an enlarged prostate, aging, or ejaculation. Conversely, certain conditions may make PSA levels low, such as certain herbal medicines or supplements, drugs to treat BPH, or obesity.
MRI (magnetic resonance imaging): A procedure that uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the body. The procedure is similar to a CT scan, except the MRI does not deliver radiation.
Transrectal ultrasound: A procedure in which a small probe, about the size of a finger, is inserted into the rectum to check the prostate. The probe is used to bounce high-energy sound waves (ultrasound) off internal tissues or organs, which create a picture of echoes (called a sonogram). A transrectal ultrasound may be used during a biopsy procedure.
A transrectal or transurethral biopsy: A procedure to remove cells, fluid, or tiny tissue samples from the prostate for viewing under a microscope by a pathologist. The pathologist will check the tissue sample to see if there are cancer cells, and then determine the Gleason score (see Gleason Grading System below).
The prognosis for prostate cancer depends on the histologic grade of the tumor, the PSA level, and whether it is confined within the prostate gland. Once the tumor penetrates the capsule and involves adjacent tissues or lymph nodes, eradication is unlikely. The Gleason system is most commonly used to grade the tumor histology. It has a range of scores between 2 and 10; scores of 2 to 4 indicate well-differentiated (low-grade), 5 to 7 moderately differentiated, and 8 to 10 poorly differentiated (high-grade) malignancies. Either the TMN or the Whitmore Jewett (W-J) staging system is used to estimate anatomically the size and extent of spread of the tumor. TMN stage T3 or W-J stage C and D cancers extend beyond the capsule of the prostate and usually cannot be completely removed. It is common for the stage to be more advanced at surgical resection than the original estimate. The diagnosis of cancer is confirmed by needle biopsy using transrectal ultrasonography of the prostate to direct the sampling of six anatomic zones (sextant) and any suspicious areas. PSA level, chest radiograph, radionuclide bone scan, and computed tomography (CT) scanning may be used to estimate the stage before planning treatment.
Tests to determine the stage of prostate cancer
If prostate cancer is found after a biopsy, tests may be conducted to determine if the cancer cells have spread beyond the prostate to other parts of the body. The process used to find out if, and how far, the cancer has spread is called staging. It is important to know the stage of the cancer - in other words, how far the cancer has progressed or is likely to progress - in order to plan treatment. The following tests and procedures may be used in the diagnostic and staging process:
Bone scan: A procedure to check if there are rapidly dividing cells, such as cancer cells, in the bone. A very small amount of radioactive material is injected into a vein and travels through the bloodstream. The radioactive material collects in the bones and is mapped by a scanner.
PET/CT (CAT) scan: A procedure in which a small amount of radioactive glucose (sugar) is injected into a vein, and a scanner is used to trace and create computerized pictures of the solution inside your body. The procedure is painless and has no side effects.
MRI (magnetic resonance imaging): A procedure that uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the body. The procedure is similar to a CT scan, except the MRI does not deliver radiation.
Pelvic lymphadenectomy: This is a procedure performed during the time of surgery to remove the lymph nodes in the pelvis. The lymph nodes are then examined under a microscope to see if they contain cancer.
Stages of prostate cancer
Prostate cancer is divided into categories - or stages - based on the size and spread of cancer beyond the prostate and into other places in the body (metastasis), such as the lymph nodes, blood, or other organs.
The clinical stages of prostate cancer are:
Stage I: Cancer is found in the prostate only. It generally does not extend beyond one half of one side of the prostate. At this stage the cancer tumor cannot be felt during a digital rectal exam and is not visible by imaging. It is usually found accidentally during other procedures, such as a biopsy, surgery, or PSA test.
Stage II: Cancer is more advanced than in stage I, but has not spread beyond the prostate. At stage II the cancer has spread to more than one-half of one side of the prostate. Tumors at this stage are usually not found by a digital rectal exam or made visible by imaging tests.
Stage III: Cancer has spread beyond the outer layer of the prostate to nearby tissues. Cancer may be found in the glands that produce semen (seminal vesicles).
Stage IV: Cancer has spread beyond the prostate and seminal glands to lymph nodes or nearby tissues in the rectum, bladder, or pelvic wall. Stage IV cancer may be found in other parts of the body, such as the liver or lungs. Metastatic prostate cancer often spreads to the bones.
What are the causes of prostate cancer?
Nobody is really sure of what the specific causes are. There are so many possible factors, including age, race, lifestyle, medications, and genetics, to name a few. Age
Age is considered as the primary risk factor. The older a man is, the higher is his risk. Prostate cancer is rare among men under the age of 45, but much more common after the age of 50.
Genetics
Statistics indicate that genetics is definitely a factor in prostate cancer risk. It is more common among certain racial groups - in the USA prostate cancer is significantly more common and also more deadly among Afro-Americans than White-Americans. A man has a much higher risk of developing cancer if his identical twin has it. A man whose brother or father had/had prostate cancer runs twice the risk of developing it, compared to other men.
Studies indicate that the two faulty genes - BRCA 1 and BRCA 2 - which are important risk factors for breast cancer and ovarian cancer, have also been implicated in prostate cancer risk.
In a study scientists found seven new sites in the human genome that are linked to men's risk of developing prostate cancer.
Faulty BRCA2 gene linked to aggressive form of prostate cancer - researchers at the The Institute of Cancer Research, UK, reported in the Journal of Clinical Oncology (April 2013 issue) that men who have inherited the faulty BRCA2 gene are more likely to have the faster-spreading type of prostate cancer. The scientists say these men should receive treatment immediately after diagnosis with surgery or radiation therapy, rather than receive the "watchful waiting" approach.
Senior author Ros Eeles wrote that experts have already known that those with the faulty BRCA2 gene have a higher risk of developing prostate cancer. This is the first large study to demonstrate that the faulty gene is also linked to a faster spread of the disease and poorer survival.
This new discovery will make some health authorities around the world rethink their policies and procedures. In the United Kingdom, the National Health Service offers the same prostate cancer treatment for both carriers and non-carriers of the faulty BRCA2 gene.
Prof. Eeles said "It must make sense to start offering affected men immediate surgery or radiotherapy, even for early-stage cases that would otherwise be classified as low-risk. We won't be able to tell for certain that earlier treatment can benefit men with inherited cancer genes until we've tested it in a clinical trial, but the hope is that our study will ultimately save lives by directing treatment at those who most need it."
Diet
A review of diets indicated that the Mediterranean diet may reduce a person's chances of developing prostate cancer. Another study indicates that soy, selenium and green tea, offer additional possibilities for disease prevention - however, a more recent study indicated that combination therapy of vitamin E, selenium and soy does not prevent the progression from high-grade prostatic intraepithelial neoplasia (HGPIN) to prostate cancer. A diet high in vegetable consumption was found in a study to be beneficial.
A US pilot study on men with low risk prostate cancer found that following an intensive healthy diet and lifestyle regime focusing on low meat and high vegetable and fruit intake, regular exercise, yoga stretching, meditation and support group participation, can alter the way that genes behave and change the progress of cancer, for instance by switching on tumor killers and turning down tumor promoters.
Other studies have indicated that lack of vitamin D, a diet high in red meat may raise a person's chances of developing prostate cancer.
A study published in the journal Clinical Cancer Research suggests vitamin D deficiency may predict aggressive prostate cancer.
Medication
Some studies say there might be a link between the daily use of anti-inflammatory medicines and prostate cancer risk. A study found that statins, which are used to lower cholesterol levels, may lower a person's risk of developing prostate cancer.
Obesity
A study found a clear link between obesity and raised prostate cancer risk, as well as a higher risk of metastasis and death among obese people who develop prostate cancer.
Sexually transmitted diseases (STDs)
Men who have had gonorrhea have a higher chance of developing prostate cancer, according to research from the University of Michigan Health System.
Agent Orange
Veterans exposed to Agent Orange have a 48% higher risk of prostate cancer recurrence following surgery than their unexposed peers, and when the disease comes back, it seems more aggressive, researchers say. Another study found that Vietnam War veterans who had been exposed to Agent Orange have significantly increased risks of prostate cancer and even greater risks of getting the most aggressive form of the disease as compared to those who were not exposed.
Enzyme PRSS3 linked to aggressive prostate cancer
Scientists from the Mayo Clinic, Florida, reported in Molecular Cancer Research that PRSS3, an enzyme, changes the environment of prostate cancer cells, making the cancer much more likely to metastasize.
Senior researcher, Evette Radisky, Ph.D., said "This molecule is a protease, which means it digests other molecules. Our data suggests PRSS3 activity changes the environment around prostate cancer cells - perhaps by freeing them from surrounding tissue - to promote malignancy and invasiveness. I don't think PRSS3 is the only factor involved in driving aggressive prostate cancer, but it may be significant for a certain subset of this cancer - the kind that is potentially lethal."
Chronic prostate inflammation tied to nearly double risk of prostate cancer - a study reported in the journal Cancer Epidemiology, Biomarkers & Prevention, finds that compared to men with no such signs, men with chronic inflammation in non-cancerous prostate tissue may have nearly double the risk of developing prostate cancer.
Vasectomy linked with aggressive prostate cancer risk - In the largest and most comprehensive study of its kind, researchers from Harvard School of Public Health in Boston, MA, find that vasectomy is associated with a small increased risk of prostate cancer, and a larger increased risk for advanced or lethal prostate cancer.
Prostate cancer: high cholesterol, triglyceride levels may raise risk of recurrence - Among men who have surgery for prostate cancer, those who have high total cholesterol and triglyceride levels - two types of fat found in blood - may be at increased risk of disease recurrence. This is according to a study published in the journal Cancer Epidemiology, Biomarkers & Prevention.
Prostate Cancer Differential Diagnosis
The principal diagnostic mandates for the practitioner who screens patients for prostate cancer are (1) differentiation of prostate cancer from benign prostatic disease and (2) detection of prostate cancer when it is still localized to the gland, a clinical circumstance most amenable to curative therapy. The greatest diagnostic dilemma is presented by the asymptomatic patient with elevated PSA values. Benign prostatic hyperplasia and acute or chronic prostatitis may elevate the PSA. Indeed, up to 20% of men with biopsy-proven BPH may have abnormal PSA levels. In addition, some experts believe that occult prostate cancer detected by elevated PSA values may be biologically unaggressive and represent little threat to survival. Nevertheless, the PSA determination remains at the heart of accepted screening practice for prostate cancer. In the event that PSA alone is elevated, TRUS is recommended. If TRUS examination is normal, random biopsies of the prostate are often performed if the PSA level is greater than 4 ng/ml. If TRUS examination reveals abnormal (hypoechoic) areas, directed biopsies of these areas are performed. If biopsies do not reveal cancer, follow-up with annual PSA determination and DRE is continued.
Annual PSA screening should be performed in conjuction with DRE. DRE may detect 16% to 20% of prostate cancers in men whose PSA level is normal. In any circumstance in which a suspected palpable prostatic abnormality is perceived, TRUS and biopsy should be sought. Such abnormalities may include a nodule in one or both lobes of the prostate, asymmetry of the prostate, or induration of a lobe of the prostate. It is a daunting realization that up to 50% of apparently localized palpable abnormalities of the prostate ultimately shown to be cancer will be upstaged and extend beyond the capsule of the gland.
The presence of prostatic symptoms offers little assistance in differentiating patients with BPH from those with prostate cancer. PSA and DRE have some value in making this distinction. Cancer is more probable in men with elevated PSA levels or a palpable abnormality of the prostate. The emergence of symptoms of bone pain, weight loss, renal dysfunction, or anemia has little diagnostic value for prostate cancer. If prostate cancer is demonstrated in the setting of these symptoms, advanced, incurable disease is the rule.
Prostate Cancer Treatment
A primary consideration when choosing the method of treatment for prostate cancer is the likelihood of the tumor shortening the patient's life or producing significant symptoms. At present there are no definitive randomized trials directly comparing the effectiveness of various treatments for localized prostate cancer. In the absence of compelling evidence, the decision to select surgical or radiation therapy is often influenced by the specialty of the treating physician.
If the cancer is localized to the prostate, treatment should prevent future spread while maintaining bladder, bowel, and sexual functions. If the patient has a low-grade confined tumor (Gleason 2-5) and his expected life span is less than 10 years, "watchful waiting" is a reasonable approach. This strategy may not be appropriate for men under age 65, as their cancer is more likely to become symptomatic. Moderate and poorly differentiated tumors (Gleason 6 to 10) are more likely to become clinically significant. If the patient's expected life span is more than 10 years, radical prostatectomy and radiation therapy are effective treatments in confined prostate cancer. Radical prostatectomy has a 0.4% mortality rate, and 30% to 70% of patients maintain sexual functioning. Urinary incontinence develops in 5% to 30% of patients after surgery. After successful surgery the PSA level should remain undetectable. Traditional external beam radiation therapy, three-dimensional (3D) conformational treatment planning with highly focused beam therapy, and the implantation of radioactive seeds into the prostate (brachytherapy) are options for the patient who is unwilling to undergo surgery. Acute complications of traditional irradiation include proctitis, cystitis, urinary retention, and scrotal edema in 30% to 50% of patients. Chronic proctitis, diarrhea, and cystitis occur in a small percentage given radiation therapy, but 50% are impotent at 7 years following treatment; 3-D conformational radiation therapy and brachytherapy have a lower rate of side effects than standard external beam therapy.
Radiation therapy is preferred if complete surgical removal of the tumor is unlikely. A Gleason score of 7 or higher, a PSA level greater than 20, or evidence the tumor has penetrated the prostate capsule (stage T3) is an indication for primary radiation therapy.
For men who present with prostate cancer that has already metastasized to lymph nodes or other sites, palliative therapy is all that can be offered. Prostate cancer spreads locally to the seminal vesicles and base of the bladder. The obturator and hypogastric lymph nodes are often involved. Common sites of osteoblastic bony metastases are the spine, pelvis, ribs, sternum, and skull. The lung, liver, and adrenal gland are sites of visceral metastases. Prostate cancer is influenced by androgens, and about 85% of patients have an objective response to androgen removal. Hormonal therapy delays the progression of cancer and provides local control for patients with advanced local disease or high-grade tumors confined to the prostate. Androgen removal is also beneficial if the PSA level is steadily rising, or when nodal or distant metastases are present. Treatment options include orchiectomy, gonadotropinreleasing hormone agonists (leuprolide/Lupron, goserelin/Zoladex), agents that interfere with androgen binding to cellular receptors (flutamide/Eulexin, bicalutamide/Casodex), and adrenal steroid inhibitors (ketoconazole, aminogluthetimide). A common combination often referred to as total androgen blockade combines a gonadotropin-releasing hormone agonist and an androgen receptor blocker. Eventually prostate cancer becomes androgen insensitive, and may paradoxically respond for a short time to discontinuing androgen blockade. Chemotherapy has very limited benefit for disease that has become resistant to hormone therapy. Local irradiation can achieve temporary control with symptoms of soft tissue or bony metastases.
What are the possible complications of prostate cancer?
Metastasis - the cancer can spread to other parts of the body through the bloodstream or the lymphatic system to other organs or bones. If the cancer spreads to the ureters, the tubes that carry urine from the kidneys to the bladder, there is a risk of serious kidney problems.
If the cancer spreads to the bones, there may be pain and fractures. Doctors say that when prostate cancer has spread to other parts of the body, it can no longer be cured, but may possibly be controlled.
Incontinence - the prostate cancer itself, or treatments can cause urinary incontinence.
Erectile dysfunction - the prostate cancer or prostate cancer treatment can lead to erectile dysfunction - the inability to achieve a penile erection, or maintain one.
Metabolic factors - a man's risk of dying from prostate cancer is much higher if he has high blood pressure, raised blood sugar levels, high blood lipid levels, and a high BMI (body mass index), which collectively are known as metabolic factors. This was reported in the journal Cancer (October 2012 issue) by scientists from Umea University in Sweden.
Prostate Cancer Management
There is no known preventive therapy of prostate cancer. A large national cooperative trial of the daily administration of finasteride, an inhibitor of 5α-reductase which converts testosterone to the multifold more potent dihydrotestosterone, is currently in progress. The study seeks to determine whether finasteride reduces the prevalence of biopsy-proven prostate cancer in men 55 years of age or older who have taken the drug for 7 years.
The election of specific therapy for prostate cancer requires the histopathologic diagnosis of the disease. Because the therapies of prostate cancer may induce important disabilities or toxicities, the ensurance of the presence of malignancy is paramount. In addition, the occasional identification of atypical forms of prostate cancer (e.g., sarcomas or small cell tumors) may warrant the avoidance of hormone ablation therapies in favor of other management approaches.
It is important that the management of prostate cancer is linked to the presenting stage of the disease (Tables 97-1 and 97-2). In general, surgery or radiation therapy is applied to disease that is still confined to the prostate gland. For cancer that has transgressed the capsule of the gland, spread to pelvic lymph nodes, or disseminated to bone or other organs, hormonal ablation or systemic chemotherapy is often used. For patients anticipating receipt of local therapy for prostate cancer, staging pelvic lymphadenectomy is recommended. This procedures has no intrinsic therapeutic value but provides important evidence of disease dissemination to pelvic (obturator and iliac) lymph nodes and ultimately to occult distant sites.
Patients commonly have clinically occult prostate cancer detected on pathologic examination of tissue obtained during transurethral resection of the prostate for BPH (T1a or T1b; stage A) or following needle biopsy of the prostate following elevated PSA level (T1c; stage A).
For stage A1 (T1a) prostate cancer, progression of disease is infrequent and removal of the prostate has not been shown to provide a survival benefit. Observation is a reasonable approach in such individuals unless the patient is young (<60 years of age). If the patient is otherwise expected to live many additional years, radiation may eradicate microscopic residual disease.
For stage A2/B (T1b, T1c, T2) prostate cancer, radical prostatectomy and irradiation (external beam with or without interstitial therapy) are essentially equivalent therapies. Adequate comparisons of radiotherapy versus surgery that provide convincing evidence of survival differences in balanced patient samples have not been performed. Both therapies can cause substantial morbidities: prostatectomy is accompanied by risks of anesthesia and surgical complication, urinary incontinence, and/or sexual impotence; radiation may induce prolonged proctitis, cystitis, urethral stricture, and/or sexual impotence. Procedures with potentially less morbidity and results equivalent to prostatectomy or external beam radiotherapy include transperineal brachytherapy or cryosurgery. Data about long-term overall and disease-free survivals for these latter techniques are pending. In addition, these techniques require comparison in clinical trials to the standard management of early-stage prostate cancer before they may be accepted as a standard of care.
Stage C (T3) prostate cancer represents a substantial therapeutic predicament for the treating physician. Attempted radical prostatectomy often reveals evidence of extracapsular extension of cancer (pathologic stage C). Clinical examination of the prostate may also suggest extracapsular extension (clinical stage C). The best therapy for this stage is unknown, and local therapies are regarded as beneficial to a minority of such patients, since most are destined to relapse. Surgery appears to have limited effectiveness in this setting due to a high frequency of positive surgical margins following attempted resection.
Radiation is not uncommonly applied to patients with stage C cancer, either alone or as adjunct to surgical resection. Hormonal ablation is frequently employed, particularly in the elderly individual for whom surgical management or radiation therapy risks serious morbidity.
Stage D1 (any T, N1-3) is characterized by the presence of metastases to pelvic lymph nodes. The detection of pelvic lymph node metastases defines an expectation of disseminated prostate cancer within 5 years. Currently controversy exists about the need for early or delayed therapy in patients with stage D1 disease. It has not been definitively shown that patients have any detrimental survival outcome with delay of hormonal ablation therapy until the appearance of disseminated disease. Consequently, expectant observation is a common approach following pelvic lymphadenectomy and detection of lymph node metastasis.
Stage D2 prostate cancer encompasses metastatic disease to all extrapelvic sites. This stage of cancer is classically managed in its initial phases with androgen ablation. Bilateral orchiectomy remains the standard against which all alternative hormone ablation methods are compared. The advantages of orchiectomy include accomplishment of androgen ablation with a single procedure, immediate effect, and avoidance of continued therapy to sustain its effectiveness. No competing ablative therapy has been shown to exceed orchiectomy for long-term effectiveness. As an alternative, prostate cancer patients with metastatic disease may elect "total androgen blockade" (Table 97-3). Total androgen blockade utilizes a parenterally administered luteinizing hormone-releasing hormone (LHRH) agonist (goserelin acetate or leuprolide acetate) plus an orally administered antiandrogen (flutamide or bicalutamide). The LHRH agonist diminishes LH and follicle-stimulating hormone blood levels and ultimately yields castrate blood levels of androgens. The antiandrogens block androgen receptors and thereby block the modest androgenic contribution from adrenal hormone synthesis. Although the major hormone ablation therapies may accomplish dramatic tumor regression, symptom relief, and perhaps survival benefits, most patients are destined to develop resistance to such therapies. Resistance to hormone ablative therapy (i.e., hormone refractory) has ominous meaning insofar as restoration of antitumor response with alternative therapies occurs in a minority of patients, and survival expectation is materially truncated. Alternative hormone ablation therapies include diethylstilbestrol, aminoglutethimide with hydrocortisone, flutamide withdrawal, or ketoconazole.
Chemotherapy is reserved for those patients who are hormone refractory (defined as failure to manifest antitumor response following one or two hormone ablative therapies). Objective antitumor responses are infrequent with chemotherapy, and survival is rarely impacted. Agents that have shown some utility include estramustine, paclitaxel (Taxol), vinblastine, doxorubicin, cisplatin, and etoposide.
Important palliative approaches for patients with symptomatic bone metastases include radiopharmaceuticals such as strontium 89 (Metastron). Antitumor agents with some promise in the treatment of advanced prostate cancer include suramin, an antitrypanosomal agent that inhibits growth factor binding to receptors on prostate cancer cells.
When to Refer
The internist engaged in general practice is likely to have ample opportunity to engage in screening procedures (PSA determination and DRE) leading to the detection of prostate cancer. Indeed, the detection of prostate cancer that is restricted to the gland is a clinical diagnostic challenge where the internist may have important impact on the survival of individual patients and ultimately on the public health. PSA determination and DRE remain the gold standards for screening. It behooves the clinician to become proficient in prostate examination by DRE. Abnormalities of the gland (asymmetry, localized induration, nodules) should be recognizable and, whether or not the PSA value is elevated, lead to prompt referral to a urologist for confirmation and further diagnostic evaluation. In addition, any PSA elevation greater than 4 ng/ml in a man over 40 years of age should prompt consultation with a urologist. Although prostate cancer is extremely uncommon in individuals younger than 50 years of age, elevation of PSA level in such men, particularly if a family history of prostate cancer in a first-degree relative is provided or if the individual is black, requires consultation with a urologist.
The primary care internist may only occasionally be confronted with a young patient with a scrotal mass. In general, most acute clinical conditions of the testis or its adnexae may be differentiated by physical examination and by the judicious application of noninvasive ultrasonography. However, the clinician must remain aware of the frequency of testicular cancer as a cause of testicular disease, of the effectiveness of therapy, and of the necessity for early recognition and management of this rapidly growing malignancy. Transscrotal biopsy of any testicular lesion should be avoided at all cost, and prompt referral to a urologist should be sought. The internist involved in the care of an individual recently treated for germ cell cancer of the testis must be prepared to pursue a rigorous follow-up schedule (monthly for the first year of follow-up, every 2 months for the second year, with a more liberal schedule only after 2 years of uneventful follow-up). The patient who has completed treatment for germ cell cancer may face delayed clinical problems, including (1) late recurrence (greater than 2 years after remission) of cancer, particularly to the central nervous system or the opposite testis, (2) diminished fertility due to RPLND or chemotherapy, and (3) prolonged toxicity of chemotherapy (e.g., bleomycin lung injury, etoposide-related acute leukemia). Early referral for evaluation of suspected recurrent cancer may be lifesaving, since aggressive salvage therapy may cure many patients.


