Herceptin found to improve long-term survival of HER2-positive breast cancer patients
VCU Massey Cancer Center physician-researcher Charles E. Geyer, Jr., M.D., was the National Protocol Officer for one component of a large national study involving two National Cancer Institute (NCI)-supported clinical trials that demonstrated that trastuzumab significantly improves the long-term survival of HER-2 positive breast cancer patients. The combined study was designed to determine the long-term safety and efficacy of the drug trastuzumab, which is commonly known as Herceptin and is primarily used alongside chemotherapy to treat HER2-positive breast cancer. The combined study focused on both the overall survival rates of patients up to ten years post-treatment as well as the known and potentially harmful side effects to the cardiac system.
Published in the Journal of Clinical Oncology, the study found that Herceptin, when added to chemotherapy, improved 10-year survival from 75 percent with chemotherapy alone to 84 percent with the addition of trastuzumab. Additionally, results also demonstrated continued improvement of survival without cancer recurrence—the 10-year disease-free survival rate increased from 62 percent to 74 percent with the addition of trastuzumab. Although heart problems are recognized side effects of Herceptin, the incidence rate of such events was found to be about 3 percent and the majority of those patients recovered from the initial effects.
“We have found that when Herceptin is used in combination with chemotherapy, a patient’s survival is significantly improved,” said Geyer, who serves as a senior scientific advisor to the NSABP and at Massey is the Harrigan, Haw, Luck Families Chair in Cancer Research, associate director for clinical research and member of the Developmental Therapeutics research program, as well as professor in the Division of Hematology, Oncology and Palliative Care at the VCU School of Medicine. “There are minimal long-term side effects, and the likelihood of the cancer recurring is greatly reduced.”
The study was designed to provide much needed long-term efficacy data on Herceptin - a proven effective treatment, but one without much information on the role it plays in patients’ long-term survival.
The study combines data from two trials: NSABP B-31, led by the National Surgical Adjuvant Breast and Bowel Project (NSABP), and NCCTG N9831, led by the North Central Cancer Treatment Group (NCCTG). Each trial was designed independently to analyze overall survival rates of patients with early-stage HER2-positive breast cancer. The study specifically addressed whether or not the patient experienced a cancer recurrence and if there were any harmful side effects that would diminish favorable treatment results.
 The local principal investigator leading the NSABP B-31 trial at Massey was Harry Bear, M.D., Ph.D. Bear, who is the Dr. Walter Lawrence, Jr. Chair in Surgical Oncology, director of the Breast Health Center and medical director of the Clinical Trials Office at Massey, also serves on the Board of Directors of the NSABP Foundation, Inc.
The local principal investigator leading the NSABP B-31 trial at Massey was Harry Bear, M.D., Ph.D. Bear, who is the Dr. Walter Lawrence, Jr. Chair in Surgical Oncology, director of the Breast Health Center and medical director of the Clinical Trials Office at Massey, also serves on the Board of Directors of the NSABP Foundation, Inc.
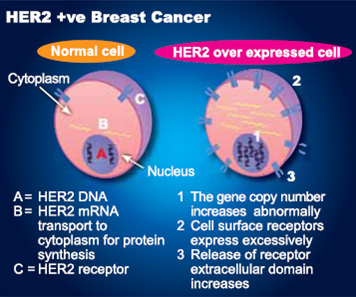
 Herceptin was approved by the Food and Drug Administration in 2006, based on the initial results of these two studies, as an adjuvant treatment for HER2-positive breast cancers, which test positive for the HER2 mutation and are often more aggressive than other types of breast cancers. HER2 - human epidermal growth factor receptor 2 - is a protein that plays a significant role in breast cancer. HER2 proteins are products of the HER2 gene and work to control the growth of healthy cells. If the proteins are overexpressed, or if the HER2 gene is amplified, the cells can grow uncontrollably and become cancerous. Approximately 15 to 20 percent of invasive breast cancers result from HER2 gene amplification or overexpression of the HER2 protein.
Herceptin was approved by the Food and Drug Administration in 2006, based on the initial results of these two studies, as an adjuvant treatment for HER2-positive breast cancers, which test positive for the HER2 mutation and are often more aggressive than other types of breast cancers. HER2 - human epidermal growth factor receptor 2 - is a protein that plays a significant role in breast cancer. HER2 proteins are products of the HER2 gene and work to control the growth of healthy cells. If the proteins are overexpressed, or if the HER2 gene is amplified, the cells can grow uncontrollably and become cancerous. Approximately 15 to 20 percent of invasive breast cancers result from HER2 gene amplification or overexpression of the HER2 protein.
Additional trials are currently underway to try to improve patient outcomes by using Herceptin in combination with various other drugs that also specifically target breast cancers with overexpressed HER2 proteins. Other trials are investigating applications of Herceptin for different cancers. For example, one study is presently investigating whether or not patients with breast cancers with lower amounts of HER2 protein might also benefit from Herceptin’s promising results.
HER2-positive breast cancer is a breast cancer that tests positive for a protein called human epidermal growth factor receptor 2 (HER2), which promotes the growth of cancer cells. In about 1 of every 5 breast cancers, the cancer cells make an excess of HER2 due to a gene mutation. This gene mutation and the elevated levels of HER2 that it causes can occur in many types of cancer — not only breast cancer. This is a gene mutation that occurs only in the cancer cells and is not a type of mutation that you can inherit from a parent.
HER2-positive breast cancers tend to be more aggressive than other types of breast cancer. They’re also less responsive to hormone treatment. However, treatments that specifically target HER2 are very effective. They include:
Trastuzumab (Herceptin). Trastuzumab, which specifically targets HER2, kills these cancer cells and decreases the risk of recurrence. Trastuzumab is often used with chemotherapy. But it may also be used alone or in combination with hormone-blocking medications, such as an aromatase inhibitor or tamoxifen. Trastuzumab is usually well tolerated, but it does have some potential side effects, such as congestive heart failure and allergic reaction.
Lapatinib (Tykerb). Like trastuzumab, lapatinib is a HER2-specific drug. Lapatinib may be effective for HER2-positive breast cancer that doesn’t respond to trastuzumab. Lapatinib is used in combination with the chemotherapy drug capecitabine (Xeloda) and the aromatase inhibitor letrozole (Femara). Lapatinib is also being studied in combination with trastuzumab. Common side effects include rash, loose stools and the potential risk of congestive heart failure.
###
HER2 is a receptor (specialized protein) on the surface of all breast cells. When a breast cell has abnormally high levels of HER2, it can drive breast cancer growth and spread. Usually, a breast cell has two copies of the HER2 gene, which controls production of the HER2 protein. About 15% to 20% of patients with invasive breast cancer have abnormally high levels of the HER2 gene and/or the HER2 protein. These cancers, referred to as HER2-positive, tend to be higher risk cancers, but certain types of chemotherapy and targeted therapy work well to treat these cancers.
Drugs that specifically block HER2 to stop the growth of cancer cells are called HER2-targeted therapies. Examples of these drugs include trastuzumab (Herceptin), lapatinib (Tykerb), pertuzumab (Perjeta), and ado-trastuzumab emtansine (T-DM1; Kadcyla). These drugs are effective against HER2-positive invasive cancers, but they are costly. Rarely, these drugs cause serious side effects, such as heart problems, liver inflammation, diarrhea, and skin problems. Therefore, it is important to accurately determine how much HER2 your cancer makes so you can receive these drugs if the cancer is HER2 positive, or avoid receiving ineffective drugs if it is HER2 negative.
The two FDA-approved methods currently used in the United States to test for HER2 are immunohistochemistry (IHC) and in-situ hybridization (ISH). IHC testing can show how much of the HER2 protein is present on the cancer cell surface, while ISH testing measures the number of copies of the HER2 gene inside each cell. There are two main types of ISH tests: fluorescence and bright-field ISH.
This study was supported by NIH grants U10-CA25224 and CA129949; NSABP grants U10-CA12027, U10-CA69651, U10-CA37377 and U10-CA69974; by the Breast Cancer Research Foundation; and by grants 35-03 from Genentech. P.A.K. received research funding from Cancer and Leukemia Group B.
###
John Wallace
.(JavaScript must be enabled to view this email address)
804-628-1550
Virginia Commonwealth University