Health Centers > Cancer Health Center > Carcinoma of the Anus: Strategies in Management
Carcinoma of the Anus: Strategies in Management
The management of anal cancer underwent an interesting transformation over the last two decades. Prior to this period, the standard definitive treatment for carcinoma of the anal canal was abdominal-perineal resection, which necessitated a permanent colostomy. The organ preservation concept appeared following the discovery of a high complete response rate from preoperative combined chemoradiation prior to abdominal-perineal resection.
The organ preservation method of treatment rapidly gained popularity and ultimately saved a large number of patients from undergoing abdominal-perineal resection and colostomy. Chemoradiation treatment itself subsequently went through an evolutionary process. Several studies have sought to define the optimal chemotherapeutic regimen as well as radiation treatment dose and fractionation. Ongoing studies attempt to define an optimal treatment regimen that yields a higher cure rate while minimizing toxicity. We review the etiology, epidemiology, and treatment regimens for anal cancer.
Carcinoma of the Anus Management
Introduction
Epidemiology
- HIV Infection
- Human Papillomavirus
- Other Causes
Prognostic factors
- Tumor Location and Size
- Lymph Node Involvement
Staging
Disease presentation
Radiation treatment
Combined modality treatment
Optimum chemotherapy regimen
Optimum radiation regimen
Treatment-related toxicities
Follow-Up
Conclusions
References
INTRODUCTION
Twenty years ago the standard definitive treatment for carcinoma of the anus was abdominal-perineal resection (APR). Organ preservation employing concomitant chemoradiation subsequently became the standard treatment for most anal cancer cases, with APR in reserve as a salvage procedure. It is noteworthy that there has never been a randomized comparison of APR with concomitant chemoradiation, nor is one likely to be done. We review the evolution of management and present treatment regimens of anal cancer.
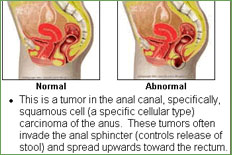
Epidermoid (squamous cell) carcinoma is the most common histological variant and represents about 80% of anal cancer cases; somewhat less common is cloacogenic (basaloid transitional cell) carcinoma. Rare types are adenocarcinoma, originating from anal glands or fistula formation, and melanoma [1].
The anal canal extends from the rectum to the anal verge and measures 2.5 to 3 cm in length. The upper border of the anal sphincter determines its superior border. The dentate line is an anatomically important structure and is located in the superior aspect of the anus, reflecting the change from squamous epithelium to transitional epithelium. Transitional epithelium, at a higher level, is replaced by the columnar epithelium of the rectum. Transitional epithelium is similar to the lining of the urinary tract. The tumor originating from this area is called cloacogenic carcinoma. The anal margin is the junction of the hair-bearing skin and the mucous membrane of the anal canal. Tumors originating from the anal margin or more distally are staged as skin cancer; this is an important distinction as they rarely involve lymph nodes or lead to distant metastases. Studies that group anal margin cancers with anal canal cancers would be grouping two cancers with differences in expected outcomes.
EPIDEMIOLOGY AND ETIOLOGY
Disease Incidence and Mortality Statistics
Carcinoma of the anal canal was about one-tenth as common as rectal cancer in a 10-year registry study in Ontario, Canada [2], and this is in general agreement with U.S. data for 2001 published by The American Cancer Society [3]. In the latter source, the annual incidence of anal cancer in the U.S. was given as 3,500 compared with 37,200 for rectal cancer. Five hundred deaths were projected, with a modest female predominance in incidence and deaths. Over 90% of patients presented with loco-regional disease and only 9% had distant metastasis [4]. The disease also increased with age [1, 2].
Carcinoma of the Anus
Anal cancer has served during the past two decades as a paradigm for the successful ...
HIV Infection
There is an increased incidence of anal cancer among HIV-positive patients [5, 6]; it is twice as common in HIV-positive as it is in HIV-negative homosexual men [7], although it is not regarded as an AIDS-defining diagnosis. Anal cancers have been increasing in young male homosexuals, and anal receptive intercourse is a risk factor in young male homosexuals and, to a lesser degree, in females [1, 2, 4-8]. The improved life expectancy in the HIV-positive population with the development of effective antiretroviral drugs may be contributing to the increased incidence of anal cancer in this population [8].
Human Papillomavirus (HPV) and Anal Cancer
Genital warts have been associated with anal cancer [2]. Papillomaviruses may cause condylomata as precursor lesions [1]. HPV type 16 is present in 30%-75% of cases of anal cancer; types 6, 11, and 18 in up to 10% [1, 9-11]. HPV appears to play a central role in disease pathogenesis, although herpes simplex virus (HSV) may play a secondary role in disease progression [10]. High-grade intraepithelial lesions or intraepithelial neoplasia may progress to invasive cancer in a similar fashion to cervical cancer in women [11, 12]. It is known that these precancerous lesions do not regress with antiretroviral therapy [13].
Other Causes of Disease
Chronic inflammatory diseases do not predispose to anal cancer development, nor is there any clear-cut association found with a history of hemorrhoids [14, 15]. Cigarette smoking, however, was found to play a role in the etiology of anogenital cancers [16-18].
PROGNOSTIC FACTORS
Tumor Location and Size
The most important prognostic factors have been defined by several studies: a tumor originating from the anal canal is more aggressive than one originating from peri-anal skin; the size of the tumor is also important, with higher T stage correlating with worse prognosis [2, 19-21]; differentiation and histologic type also play roles-epidermoid carcinoma has a better prognosis than adenocarcinoma; finally, prognosis is better in females than in males [2, 4].
Lymph Node Involvement
There is controversy concerning the adverse prognostic effect of lymph node involvement, specifically with inguinal lymphadenopathy. Moreover, in many series there is no differentiation between N1, N2, or N3 involvement. Historically, lymphadenopathy was found to carry a worse prognosis, with higher local failure rate and decreased survival, as reported in the multivariate analysis of a European Organization for Research and Treatment of Cancer (EORTC) trial [22]. Another randomized trial associated the presence of lymphadenopathy with the necessity of a higher salvage APR rate [23]. According to some investigators, younger aged patients have worse prognoses. Treatment delay may also have an adverse effect [21].
Does HIV Infection Affect Disease Outcome?
Prior to the introduction of highly active antiretroviral therapy (HAART), there was a reluctance to treat HIV-infected individuals with standard regimens of combined chemoradiation because of their poor prognoses, secondary to HIV disease itself, and fear of toxicities. These patients then had even worse prognoses. Following the introduction of HAART, several studies have shown that HIV patients can tolerate standard combined modality therapy with disease-free survival comparable with HIV-negative individuals, particularly when their CD4 counts were above 200 [24-27].
Staging
The staging system for anal cancer has undergone a long evolution. Initially, multiple staging systems were adopted by different schools. Some early surgical studies used the Dukes system, which did not reflect well the involvement of the different echelons of lymph nodes (any nodes positive were simply stage C) or the tumor size. Several others-mostly clinical staging systems-evolved thereafter, but lacked prognostic validity as well. After its adoption, tumor-node-metastasis (TNM) staging underwent substantial changes. The most recent version, published by the American Joint Committee on Cancer (AJCC), is based on clinical and surgical-pathological assessment and more precisely reflects prognostic factors, specifically the size of the tumor and the regional lymphatic spread [1, 28].
Carcinoma of the Anus
Introduction
Epidemiology
- Sexual transmission / HPV infection
- Immune suppression / HIV infection
Pathogenesis
Clinical features
Staging
Treatment
Anal canal cancer
Anal margin cancer
Anal intraepithelial neoplasia (AIN)
References
DISEASE PRESENTATION
Clinical Symptoms and Natural Progression
The most common presenting symptoms of anal cancer include pain, irritation, and bleeding in the anal area. The proper workup for anal cancer should begin with a careful physical examination including digital rectal examination and palpation of the inguinal nodal area. The nature of a suspicious lesion should be confirmed by biopsy. The disease is locally invasive and also spreads via lymphatic channels. Lymphadenopathy is clinically evident in about 20% of cases at presentation [2]. Surgical exploration, however, discloses lymph node metastasis to be present in 30%-63% of cases in various series [29]. Tumors originating above the dentate line metastasize via lymph nodes accompanying the lower hemorrhoidal veins up to the internal iliac nodes and then to the para-aortic chain. Tumors located at a lower level spread predominantly to the femoral and inguinal lymph nodes. Visceral metastasis is present in only about 10% of cases at presentation, with the most common sites of distant metastasis being lung and liver [1, 2, 4].
Diagnostic Workup
Computerized tomography scans of the chest, abdomen, and pelvis help define the internal extension of the infiltrating tumors, as well as enlargement of the pelvic nodes and distant metastasis. Endorectal ultrasound is a valuable tool for assessing the extent of tumor infiltration, also, to some degree, peri-anal lymph node involvement [30]. The role of magnetic resonance imaging is undefined for anal cancer evaluation, although from available experience with rectal cancer, this diagnostic method may warrant further investigation.
Surgical Treatment
Surgery was the primary treatment for anal cancer 20 years ago. Local resection was usually done for cancer of the anal margin, which behaves similarly to skin cancer, and was sometimes used for smaller lesions of the anal canal. Abdominal-perineal resection with colostomy was the preferred surgical procedure for most major cancers of the anal canal, and it remains the surgical procedure of choice for local recurrence. This surgical technique was adopted for the purpose of achieving adequate margins of resection; it necessitated the sacrifice of the anal sphincter, but it also enabled removal of perirectal and superior hemorrhoidal lymph nodes. Some centers advocated deeper pelvic node dissection, including the internal and external iliac nodes, but failed to show any improvement in survival. Some surgeons perform inguinal lymphadenectomy for palliative purposes for synchronous groin metastasis and curative inguinal dissection for metachronous metastasis. Surgical treatment alone resulted in local failures in about 27%-47% of cases, according to various series [28, 31, 32]. According to some series, 5-year survival rates were about 50%-70%, with higher survival rates in patients with local excisions of their tumors. This was likely attributable to earlier stage of disease [31, 32]. The majority of adenocarcinomas are still treated with the radical surgical approach.
Although there have been no randomized trials comparing surgery with radiation treatment or with combined chemoradiation, based on multiple studies and clinical experience for the last 20 years, there has been a substantial change in the management of epidermoid anal carcinomas, with more patients undergoing nonsurgical treatment [4].
RADIATION TREATMENT AS A SOLE MODALITY
External Beam Radiation Therapy
The advantage of radiation therapy is that it combines preservation of anal anatomy and function with good long-term disease-free survival. Patients are curable with organ preservation in 65%-75% of cases, which compares favorably with the survival rates reported in a series in which patients were treated by APR [31, 32]. In most series, complete response of the tumor was achieved in 75%-85% of cases [33-37].
Radiation therapy had been employed for anal cancer as early as the 1920s; early data indicated that anal cancers responded to radiation treatment [38, 39]. Radiation treatment was used for only a few patients in the early period, however, because the lower energy beams available gave an unfavorable dose distribution with limited penetration of deeper tissues and substantial perineal skin toxicity; this technique was not widely adopted. Following the advent of cobalt machines in the 1950s with higher energy beams, several studies were published on the treatment of anal cancer. Staging was less sophisticated at first, and patient groups were heterogeneous in this period.
Tumors of the Small and Large Intestines
Hereditary nonpolyposis colorectal carcinoma
Anal Carcinoma
Benign tumors of the large intestine
Malignant tumors of the large intestine
Benign tumors of the small intestine
Carcinoid Tumors of the Small and Large Intestines
Malignant Tumors of the Small Intestine
Anorectal Cancer
Carcinoma of the anus
Surgical Management of Colorectal Cancer
Colorectal cancer management
Risk factors for colorectal Neoplasia
Colorectal cancer Risk Factors
Colorectal cancer General Considerations
Colonic or Rectal cancer Treatment
Martenson and colleagues from the Mayo Clinic published a more recent retrospective study of 18 patients treated with external beam radiation only for stage T1 and T2 tumors. They showed a high tumor control rate with 100% freedom from local recurrence, and a 94% 5-year survival rate. Radiation doses used in this study were higher than those used previously, reaching 67 Gray (Gy) total [33]. Researchers from Memorial Sloan Kettering Cancer Center suggested that a select group of patients with small lesions were candidates for initial excisional biopsy followed by postoperative radiation with a limited dose of 30 Gy, this being adequate for disease control [40].
Role of Interstitial Radiotherapy
Interstitial radiation therapy has been suggested as a beneficial option for treatment of patients without lymph node involvement. Compared with external beam therapy, it has the potential to deliver a high dose to a more restricted tissue volume. In earlier times, radium needles were used for implanting in more accessible tumors. Iridium-192 (192Ir) sources have been used more recently, and single-plane implantation methods are preferred over multiplanar implants to avoid significant tissue toxicities, including necrosis. Interstitial implantation is used more often in some European institutions. Papillon et al., from France, treated 221 patients with cobalt-60 therapy combined with interstitial implants. They used a combination of perineal and sacral fields and delivered about 45 to 50 Gy of radiation; thereafter, the patients underwent a 2-month rest to recover from side effects and also to permit regression of the tumor. The patients were then implanted with 192Ir sources. They achieved good tumor response rates, with a 5-year survival rate of 65% and an anal preservation rate of 61%, preserving the anus and retaining normal sphincter function in more than 90% of surviving patients. The main concern was tissue necrosis, which appeared to some degree in more than 20% of cases. This can be associated with soft tissue infection and poor sphincter function [41-44]. In a more recent study, researchers from the United Kingdom reported the results of the treatment of 79 patients with an orthovoltage external beam radiation treatment combined with 192Ir implantation. The patients were followed for 10 years. Complete response rates were good: 90% for stage T1 and 78% for stage T2. No significant treatment-related toxicity was reported. Local failure rate was 22%, and 48% of failed patients were surgically salvaged [45].
COMBINED MODALITY TREATMENT
Retrospective Data on Combined Chemoradiation
In the 1970s, Nigro pioneered preoperative combination chemoradiation therapy to convert unresectable cases to resectable cases. There was no surgical pathological evidence of tumor found in three out of three patients treated with this approach in an early report. This led to the concept of definitive radiation therapy combined with chemotherapy [46]. There are now multiple published clinical series on combined chemoradiation[47-52]. Most reported studies have utilized a treatment regimen similar to the original Nigro protocol that combined radiation treatment with 5-fluorouracil (5-FU) and mitomycin. However, the radiation dose delivered was moving from Nigro's 30 Gy [55] towards 45-50 Gy [49, 57, 58].
One of the largest series is from Princess Margaret Hospital in Canada. Cummings et al. reviewed 192 patients treated with various approaches: radiation therapy alone, uninterrupted combined chemoradiation with 5-FU and mitomycin, split-course combined treatment with 5-FU and mitomycin, and split-course treatment combined with 5-FU alone. The treatment technique included external beam radiation to a lower pelvic field with a radiation dose of 45 Gy-55 Gy; occasionally, a boost was delivered with a perineal field or a posterior arc. Brachytherapy alone or in combination with external beam radiation treatment was seldom employed. The 5-year disease-free survival rate in the radiation treatment alone group was 68%; this rate was 64% when radiation was combined with 5-FU alone and 76% when radiation was combined with 5-FU and mitomycin. Local tumor control was also highest in the combined radiation, 5-FU, and mitomycin group, reaching 86%. Anorectal function was preserved in 88% of patients whose primary disease was controlled and in 64% of cases overall. Involvement of regional lymph nodes did not affect survival in this series. The combined treatment regimen with 5-FU and mitomycin and an uninterrupted radiation course was, however, associated with the highest morbidity. Split course and decreased fraction size was therefore explored, which resulted in decreased toxicity without significantly affecting tumor control. The omission of mitomycin C adversely affected local tumor control (p = 0.001) [49, 59].
In a Swedish study, patients with squamous cell histology were treated with split-course radiation to a total dose of 65 Gy combined with bleomycin given daily during the first 15 treatments. Excellent outcome was achieved in terms of organ preservation and disease-free survival [50].
Prospective Trials Examining a Combined Regimen
Based on several promising single-institution experiences, several randomized trials were conducted that compared radiation therapy alone with combined chemoradiation. The Radiation Therapy Oncology Group (RTOG) undertook trial 8314 exploring the effectiveness of combined chemoradiation therapy using a 5-FU and mitomycin regimen; this proved effective, with 73% overall survival at 3 years [61]. The United Kingdom Coordinating Committee on Cancer Research (UKCCCR) trial randomized 585 patients to receive radiation therapy alone or combined with continuous 5-FU during the first and final week of radiation treatment and mitomycin also on day 1 of the first course. Good responders (>50% tumor regression) received an additional radiation boost; failed responders (<50% tumor response) underwent surgical resection. This study revealed a 59% failure rate in the radiation alone arm versus 36% in the combined modality arm (p < 0.0001). There was also a 46% reduction in risk of recurrence in the combined modality arm. Overall survival was somewhat better in the combined modality arm (65% versus 58%), although it did not reach statistical significance [62]. An EORTC study also investigated the role of combined treatment modality, randomizing 110 patients. The radiation treatment technique included an initial pelvic field treated to 45 Gy with evaluation of patients after 6 weeks. An additional boost was given with 20 or 15 Gy in case of partial or complete response, respectively. In the combined group, 5-FU, 750 mg/m2/d, was delivered by continuous infusion, and a single 15-mg dose of mitomycin was given on day 1. The complete response rate was greater in the combined modality arm at 80% versus 54%. There was also significantly greater loco-regional control at 5 years, by 18% (p = 0.02), and colostomy-free survival at 5 years, by 32% (p = 0.002). Overall survival was similar in both arms. No significant toxicities were associated with the combined treatment in this study [22]. The general conclusion reached was that combined modality treatment surpassed single modality treatment for most cases.
OPTIMUM CHEMOTHERAPY REGIMEN
Role of Mitomycin C
The optimum chemotherapy regimen in treating anal canal carcinoma is under investigation. Mitomycin C has been shown to be an important drug in the treatment of anal canal cancer. The data from Princess Margaret Hospital suggested that deletion of mitomycin from the combined modality treatment of anal carcinomas had serious adverse consequences in cancer control [49]. In addition, the Eastern Cooperative Oncology Group (ECOG) and RTOG conducted a multi-institutional trial that addressed the importance of mitomycin in this combined regimen. In this trial, 310 patients were treated with either radiation therapy and fluorouracil or radiation therapy, 5-FU, and mitomycin. Results showed significantly better local control for the arm that included mitomycin, with posttreatment biopsies positive in 15% of patients in the 5-FU/radiation arm versus 7.7% in the arm that included mitomycin. Colostomy-free survival (71% versus 59%, p = 0.014) and disease-free survival (73% versus 51%, p = 0.0003) were also superior in the mitomycin group despite a greater incidence of treatment-related toxicity. The radiation treatment regimen consisted of 1.8 Gy per fraction to 45 Gy, with a field reduction after 30.6 Gy, and an optional boost to 50.4 Gy. In this study, patients underwent a biopsy at 6 weeks after completion of treatment and, if positive, received a salvage 9-Gy boost of radiation combined with 5-FU and cisplatin [23]. The results of this study again showed a statistically significant difference in favor of the mitomycin arm in local control, colostomy rate, and disease-free survival at 2 years. At the reported time point of analysis, distant disease and overall survival showed a trend in favor of the mitomycin arm. The reservations concerning the use of mitomycin relate to a situation akin to the use of the spinal cord dose limit of 45 Gy; the complication of leukemia is rare, but when it occurs, it has very unfortunate consequences, since this leukemia is usually incurable and is directly attributable to the previous treatment (with potential medical-legal implications).
Is Cisplatin a Good Substitute for Mitomycin C?
A new direction in the elimination of mitomycin from the treatment regimen was the effort to substitute some other active agent for it. Cisplatin was known to be an effective radiosensitizing agent, and it had shown good results in the treatment of squamous cell carcinomas of other sites as well as metastatic anal cancer. After using it in combined chemoradiation regimens for definitive treatment of anal carcinoma, several investigators reported good disease control and colostomy-free survival that was comparable with the mitomycin regimen, and overall survival was even slightly higher [54, 63-68]. Most of these trials used higher doses of radiation than Nigro had originally employed. French investigators tested the feasibility of using cisplatin for anal canal cancer in a phase II trial [69]. Two induction and two concomitant cycles of 5-FU, 800 mg/m2, and cisplatin, 80 mg/m2, were delivered in this study. Treatment tolerance was acceptable. Complete response was found in 96% of patients 2 months after completion of chemoradiation treatment. Building upon the available cisplatin and 5-FU data, the ECOG 4292 study enrolled 19 patients in a phase II trial to receive 59.4 Gy of radiation in 33 fractions with a 2-week break after 36 Gy. This was combined with 5-FU and cisplatin. This trial showed an overall disease response of 95%, and complete responses of 68%. One patient with stable disease and one patient with partial response at completion of treatment subsequently had complete disappearance of the tumor at more than 8 weeks after treatment. The toxicity profile was acceptable [63]. The Cancer and Leukemia Group B (CALGB) conducted a trial for locally advanced disease (T3-4) with induction chemotherapy utilizing 1,000 mg/m2 of 5-FU and 100 mg/m2 of cisplatin followed by combined chemoradiation with concurrent 5-FU and mitomycin. Complete response was found in 80% of cases, colostomy-free survival in 56% of patients, and overall survival was 78% [70]. Present evidence suggests that 5-FU can be optimally combined with cisplatin rather than mitomycin in the multimodal treatment of anal carcinomas. Currently, a randomized RTOG phase III trial is comparing 5-FU and mitomycin C with 5-FU and cisplatin in a multimodal approach combined with radiotherapy.
OPTIMUM RADIATION REGIMEN FOR COMBINED MODALITY THERAPY
What is the Optimal Radiation Treatment Dose?
Nigro originally used rather modest doses to obtain tumor responses with combined modality therapy, and the Memorial Sloan Kettering group used excisional biopsy and 30 Gy to control small lesions. It is probably true that a dose of little more than 30 Gy is adequate to gain control of an area where there is only risk of microscopic tumor burden [40]. There are, however, data that suggest a dose-response relationship for control of gross tumor burden [56, 71-73]. A retrospective study of anal carcinoma patients from Massachusetts General Hospital treated with combined chemoradiation therapy with varying radiation dose showed that patients treated with < 54 Gy had a higher rate of local recurrence compared with those treated with a dose > 54 Gy. A total dose of 56 Gy with field reductions was given without any significant increase in toxicity. Doses >54 Gy were associated with improved 5-year survival (84% versus 47%, p = 0.02) and local control (77% versus 61%, p = 0.04) compared with doses <54 Gy [71-73]. Data from M.D. Anderson Cancer Center also showed a correlation between disease response and radiation dose: >55 Gy led to better disease response and control than lower doses [56]. Night et al. arrived at a similar conclusion; according to their data, the dose was an important determinant of local control in anal cancer cases; specifically, >55 Gy yielded statistically significant better local disease control than did lower doses [53].
Colorectal cancer
Colorectal Cancer definition
Presentation
Risk Factors
Epidemiology
Colorectal cancer Risk Factors
General Considerations
Incidence and Location
Variations in Incidence Within Countries
Anatomy and Pathogenesis
Diagnosis and Screening
Clinical Findings
Differential Diagnosis
Screening for Colorectal Neoplasms
Classification Systems
Treatment
Colorectal Neoplasms Treatment
Prognosis
Follow-Up after Surgery
Risk factors for colorectal Neoplasia
Prevention
References
Is a Treatment Break Advisable?
In 1992, RTOG launched a phase III study (RTOG 92-08) based on dose escalation. In this study, the chemotherapy regimen included 5-FU and mitomycin, with similar doses and administration schedule as in previous studies. The radiation dose was escalated to a total of 59.4 Gy in 33 fractions; a 2-week rest period was mandatory after 36 Gy. Preliminary analysis of this study by John and colleagues revealed similar toxicity rates with this regimen to RTOG 87-04, and also showed a significantly greater number of local failures with a high rate of colostomy compared with RTOG 87-04, 23% versus 6%. This was attributed to the 2-week treatment break and, likely, to a larger number of patients requiring a treatment break [73, 74]. This was at odds with some other data, particularly the Princess Margaret experience, which did not find an adverse effect with split-course radiation. In the next phase of this study, the mandatory treatment break was eliminated, and the colostomy rate decreased to 11%. Based on this, the more recent approach is to eliminate planned breaks.
The Latest in Prospective Randomized Trials
Utilizing concepts of dose escalation, continuous rather than split-course radiation treatment, and consideration of substitution of cisplatin for mitomycin, RTOG initiated the new trial, 98-11. Patients are being randomized to two arms comparing the conventional 5-FU and mitomycin regimen with 5-FU and cisplatin chemotherapy. One criticism that has been made is that there is an asymmetry in the way the chemotherapy is combined with radiation; in the 5-FU/cisplatin arm, two cycles of chemotherapy are given neoadjuvantly, as in the CALGB trial, and then the same chemotherapy is combined with radiation. In the 5-FU/mitomycin arm, chemotherapy and radiation are started simultaneously. Induction chemotherapy is thought to reduce tumor bulk, and a total of four cycles of chemotherapy may reduce the rate of distant metastasis. The radiation dose is taken up to 59.4 Gy for residual lesions and all T3, T4, or node-positive patients. There is no mandatory treatment break in this protocol. The study calls for optional primary site biopsy if a palpable residual disease is present at 8 weeks after the treatment. This study is ongoing, accruing patients, and its results will likely help define future therapy of anal carcinomas.
Treatment of HIV-Infected Individuals
As was previously noted, HIV infection is associated with a markedly increased incidence of anal cancer, and increasing numbers of patients present for treatment with this major comorbidity. Despite initial controversy, our current data suggest the appropriateness of standard combined regimens for anal canal cancer treatment in HIV-infected patients. There are several retrospective series addressing this issue, however, most of them included small numbers of cases [24-27]. One of the largest series came from the University of California San Francisco [26]. Hoffman and colleagues reviewed 17 cases of HIV-infected patients treated with combined chemoradiation. Radiation treatment doses ranged from 32 Gy-63 Gy, and chemotherapy was also given with conventional dose regimens, including 5-FU combined with mitomycin or cisplatin. This study showed that patients with CD4 counts more than 200 had acceptable treatment-related toxicity as well as very good disease control (all nine patients). On the other hand, patients with CD4 counts less than 200 had a markedly higher incidence of morbidities, with four out of eight patients requiring hospitalization. Satisfactory treatment outcome was observed even with this group of patients, with disease ultimately controlled in seven out of eight cases. A similarly high disease control rate and acceptable toxicity rate were reported by British colleagues when they treated HIV-associated anal canal cancer with conventional doses of radiation and chemotherapy [24]. Complete remission was obtained in 9 out of 11 patients. One patient suffered from fatal recurrent bowel obstruction. Unlike the San Francisco study, they did not detect any greater frequency of toxicity for patients with a CD4 count less than 200.
TREATMENT-RELATED TOXICITIES
The most common acute side effect of anal cancer is skin reaction with erythema, pigmentation change, and desquamation, generally grade II and III, sometimes leading to treatment interruption [21, 22, 75, 76]. Impairment of anal function depends, in large measure, on the extent of prior surgical intervention or on subsequent biopsy, and this can become a permanent problem. Interstitial brachytherapy is associated with a higher incidence of anal dysfunction as a result of tissue necrosis; this ranges from 2%-15% [41-45].
FOLLOW-UP AND SALVAGE TREATMENT
Evaluation for Treatment Response
The mainstay in tumor response assessment is relatively close clinical follow-up, repeating physical examination of the anal area at approximately 6-week intervals. Different researchers recommend different waiting periods after the radiation treatment for full response of the tumor: between 6-12 and up to 30 weeks. Early biopsy is recommended for clinically apparent tumor progression or failure to respond, however, a biopsy at 6 weeks after completion of treatment may not represent the ultimate true response of a tumor. It is known from the experience at Princess Margaret, and other centers, that anal carcinomas continue to respond up to 9 months after treatment [49]. In an alternate approach, patients were examined every 6 weeks and only those with clinical progression underwent early biopsy. The point in avoiding unnecessary biopsy procedures is to minimize the risk of soft tissue infections, tissue necrosis, and impairment of anal function. There is still controversy regarding the definition and management of residual disease. Biopsy is recommended earlier for tumor mass progression or unsatisfactory response. RTOG suggested giving an additional 9 Gy of radiation treatment for salvage, rather than resorting to APR immediately. Alternative recommendations include delivering a higher dose of radiation for larger tumors, up to 59.4 Gy, as initial treatment.
Surgical Salvage for Disease Persistence
Patients with residual microscopic foci of tumor may undergo local resection of tumor. Others with larger residual disease receive salvage APR. The overall survival data for surgical salvage at 5 years is about 30%-60% [77, 78]. Some use, as an option, a repeat combined chemoradiation treatment for salvage purposes, with comparable success. There is no randomized study that compares different salvage approaches. In the current RTOG trial, the recommendation is to give an additional combined chemoradiation treatment for persistent disease and to reserve APR for failure of this second-line treatment, as about 50% of patients can still be salvaged with organ preservation.
Treatment of Locally Recurrent and Metastatic Disease with Chemotherapy
Different regimens of salvage chemotherapy may be used for metastatic anal carcinoma, although the commonest regimens at present employ cisplatin and 5-FU [79, 80]. We may anticipate that as new chemotherapeutic agents are developing, this treatment strategy may undergo changes in the future.
CONCLUSIONS
Management of carcinomas of the anal canal has progressed from a radical surgical approach to an organ-preserving approach utilizing combined chemotherapy and radiation treatment, with high rates of success. A good option for those practicing in a setting in which protocol treatment can be made available is to enter patients on RTOG 98-11; this apparently represents the state-of-the-art treatment. Accrual of larger numbers of patients to the trial will provide greater statistical validity to conclusions, which will shape the treatment of this disease in coming years. If patients are treated outside of a protocol setting, one could consider using a chemoradiation approach in which the chemotherapy consists of 5-FU, 1,000 mg/m2, delivered by continuous infusion on days 1-4 and 29-32 combined with 10 mg/m2 mitomycin bolus on days 1 and 29. Treatment with cisplatin-containing regimens is generally considered investigational at this point, so that it would be recommended that it be given only under the protocol until results of this latest intergroup trial are known. There is evidence of a radiation dose response available; little more than 30 Gy may be needed to control areas in which there is only a risk of microscopic tumor burden. Gross tumor may require doses >55 Gy to give optimal probability of disease eradication. As in many other curative treatment situations, continuous-course irradiation apparently confers an advantage over split course radiation treatment. Tumors may continue to regress for several months after treatment. It may be wisest to follow clinically, as long as the tumor is gradually regressing, and not to biopsy or perform other surgical procedures too quickly, as this could lead to complications, such as infection, fistula, or loss of good sphincter function. Initial local failures may be salvaged by either additional chemoradiation or by abdominal-perineal resection. Chemoradiation causes generally tolerable but significant side effects, and efforts continue to retain high disease-free survival rates while mitigating side effects. Anal cancer screening and prevention is gaining more acceptances and warrants further study [81, 82].
Emory University School of Medicine, Department of Radiation Oncology, Atlanta, Georgia, USA
Richard H. Matthews, M.D., Ph.D., Harvard University, Department of Radiation Oncology, Beth Israel/Deaconess Medical Center, 330 Brookline Avenue, Boston, Massachusetts 02215, USA. Telephone: 617-232-9500, x4457 or x5628; Fax: 617-524-0643; e-mail: RichardHMatthews@aol.com
References