Health Centers > Cancer Health Center > Bladder Cancer
Bladder Carcinomas
Incidence
Bladder cancer is the second most common cancer of the genitourinary tract. It accounts for 7% of new cancer cases in men and 2% of new cancer cases in women. The incidence is higher in whites than in African Americans, and there is a positive social class gradient for bladder cancer in both sexes. The average age at diagnosis is 65 years. At that time, approximately 85% of bladder cancers are localized to the bladder; 15% have spread to regional lymph nodes or distant sites.
Risk Factors & Pathogenesis
Cigarette smoking accounts for 50% of cases in men and 31% in women (Wynder and Goldsmith, 1977). In general, smokers have approximately a 2-fold increased risk of bladder cancer than nonsmokers, and the association appears to be dose-related (Thompson and Fair, 1990; Mommsen and Aagaard, 1983). The causative agents are thought to be alpha- and beta-naphthylamine, which are secreted into the urine of smokers.
Occupational exposure accounts for 15-35% of cases in men and 1-6% in women (Matanoski and Elliott, 1981). Workers in the chemical, dye, rubber, petroleum, leather, and printing industries are at increased risk. Specific occupational carcinogens include benzidine, beta-naphthylamine, and 4-aminobiphenyl, and the latency period between exposure and tumor development may be prolonged. Patients who have received cyclophosphamide (Cytoxan) for the management of various malignant diseases are also at increased risk (Fairchild et al, 1979). Ingestion of artificial sweeteners has been proposed to be a risk factor, but several studies have failed to confirm any association (Howe et al, 1977; Najem et al, 1982; Elcock and Morgan, 1993). Physical trauma to the urothelium induced by infection, instrumentation, and calculi increases the risk of malignancy (Hicks, 1982).
The exact genetic events leading to the development of bladder cancer are unknown, but they are likely to be multiple and may involve the activation of oncogenes and inactivation or loss of tumor suppressor genes (Olumi et al, 1990). Loss of genetic material on chromosome 9 appears to be a consistent finding in patients with both low-grade, low-stage and high-grade, high-stage disease (Tsai et al, 1990; Miyao et al, 1993), which suggests that this may be an early event in bladder cancer development. Loss of chromosome 9 in multiple tumors from an individual patient supports the concept that genetic changes in bladder cancer represent a "field defect" that may occur throughout the urothelium. Additional genetic changes have been described that are specific for invasive bladder tumors. Chromosome 11p, which contains the c-Ha-ras proto-oncogene, is deleted in approximately 40% of bladder cancers (Olumi et al, 1990). Increased expression of the c-Ha-ras protein product, p21, has been detected in dysplastic and high-grade tumors but not in low-grade bladder cancers. Deletions of chromosome 17p have also been detected in over 60% of all invasive bladder cancers, but 17p deletions have not been described in superficial tumors. This finding is noteworthy because the p53 tumor suppressor gene maps to chromosome 17p. p53 alterations represent the most commonly identified genetic abnormality in human cancers, making deletion of this chromosome an important finding in muscle invasive bladder cancer.
Staging
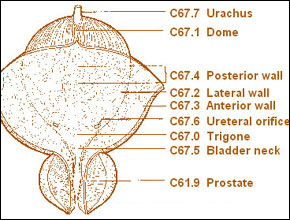 The initial staging system for bladder cancer was proposed by Jewett and Strong (1946) and was later modified by Marshall (1952). Currently, the most commonly used staging system allows for a precise and simultaneous description of the primary tumor stage (T stage), the status of lymph nodes (N stage), and metastatic sites (M stage) (American Joint Committee on Cancer, 1997). The 2 staging systems are compared in Figure 20-1. Although either provides a reasonable estimate of a given tumor's biologic potential and the need for and type of treatment, significant staging errors exist when one compares the clinical stage (that based on physical examination and imaging) with the pathologic stage (that based on removal of the bladder and regional lymph nodes). Overstaging is relatively uncommon, but clinical understaging may occur in up to 53% of patients (Skinner, 1977; Skinner, 1982; Dutta et al, 2001).
The initial staging system for bladder cancer was proposed by Jewett and Strong (1946) and was later modified by Marshall (1952). Currently, the most commonly used staging system allows for a precise and simultaneous description of the primary tumor stage (T stage), the status of lymph nodes (N stage), and metastatic sites (M stage) (American Joint Committee on Cancer, 1997). The 2 staging systems are compared in Figure 20-1. Although either provides a reasonable estimate of a given tumor's biologic potential and the need for and type of treatment, significant staging errors exist when one compares the clinical stage (that based on physical examination and imaging) with the pathologic stage (that based on removal of the bladder and regional lymph nodes). Overstaging is relatively uncommon, but clinical understaging may occur in up to 53% of patients (Skinner, 1977; Skinner, 1982; Dutta et al, 2001).
Symptoms include
Blood in your urine
A frequent urge to urinate
Pain when you urinate
Low back pain
Risk factors for developing bladder cancer include smoking and exposure to certain chemicals in the workplace. People with a family history of bladder cancer or who are older, white, or male have a higher risk.
Treatments for bladder cancer include surgery, radiation therapy, chemotherapy, and biologic therapy. Biologic therapy boosts your body's own ability to fight cancer.
Histopathology
Ninety-eight percent of all bladder cancers are epithelial malignancies, with most being transitional cell carcinomas.
The normal urothelium is composed of 3-7 layers of transitional cell epithelium resting on a basement membrane composed of extracellular matrix (collagen, adhesive glycoproteins, glycosaminoglycans) (Figure 20- 2A). The epithelial cells vary in appearance: The basal cells are actively proliferating cells resting on the basement membrane; the luminal cells, perhaps the most important feature of a normal bladder epithelium, are larger umbrella-like cells that are bound together by tight junctions. Beyond the basement membrane is loose connective tissue, the lamina propria, in which occasionally smooth-muscle fibers can be identified. These fibers should be distinguished from deeper, more extensive muscle elements defining the true muscularis propria. The muscle wall of the bladder is composed of muscle bundles coursing in multiple directions. As these converge near the bladder neck, 3 layers can be recognized: inner and outer longitudinally oriented layers and a middle, circularly oriented layer.
Types of bladder cancer
Different types of cells in your bladder can become cancerous. The type of bladder cell where cancer begins determines the type of bladder cancer. Your bladder cancer type determines which treatments may work best for you. Types of bladder cancer include:
Transitional cell carcinoma. Transitional cell carcinoma occurs in the cells that line the inside of your bladder. Transitional cells expand when your bladder is full and contract when your bladder is empty. These same cells line the inside of your ureters and your urethra, and tumors can form in those places as well. Transitional cell carcinoma is the most common type of bladder cancer in the United States.
Squamous cell carcinoma. Squamous cells appear in your bladder in response to infection and irritation. Over time they can become cancerous. Squamous cell bladder cancer is rare in the United States. It's more common in parts of the world where a certain parasitic infection (schistosomiasis) is a prevalent cause of bladder infections.
Adenocarcinoma. Adenocarcinoma begins in cells that make up mucus-secreting glands in the bladder. Adenocarcinoma of the bladder is rare in the United States.
Some bladder cancers include more than one type of cell.
Factors that may increase your risk of bladder cancer include:
Smoking. Smoking cigarettes, cigars or pipes may increase your risk of bladder cancer by causing harmful chemicals to accumulate in your urine. When you smoke, your body processes the chemicals in the smoke and excretes some of them in your urine. These harmful chemicals may damage the lining of your bladder, which can increase your risk of cancer.
Increasing age. Your risk of bladder cancer increases as you age. Bladder cancer can occur at any age, but it's rarely found in people younger than 40.
Being white. Whites have a greater risk of bladder cancer than do people of other races.
Being a man. Men are more likely to develop bladder cancer than women are.
Exposure to certain chemicals. Your kidneys play a key role in filtering harmful chemicals from your bloodstream and moving them into your bladder. Because of this, it's thought that being around certain chemicals may increase your risk of bladder cancer. Chemicals linked to bladder cancer risk include arsenic and chemicals used in the manufacture of dyes, rubber, leather, textiles and paint products.
Previous cancer treatment. Treatment with the anti-cancer drug cyclophosphamide (Cytoxan) increases your risk of bladder cancer. People who received radiation treatments aimed at the pelvis for a previous cancer may have an elevated risk of developing bladder cancer.
Taking a certain diabetes medication. People who take the diabetes medication pioglitazone (Actos) for more than a year may have an increased risk of bladder cancer. Other diabetes medications contain pioglitazone, including pioglitazone and metformin (Actoplus Met) and pioglitazone and glimepiride (Duetact).
Chronic bladder inflammation. Chronic or repeated urinary infections or inflammations (cystitis), such as may happen with long-term use of a urinary catheter, may increase your risk of a squamous cell bladder cancer. In some areas of the world, squamous cell carcinoma is linked to chronic bladder inflammation caused by the parasitic infection known as schistosomiasis.
Personal or family history of cancer. If you've had bladder cancer, you're more likely to get it again. If one or more of your immediate relatives have a history of bladder cancer, you may have an increased risk of the disease, although it's rare for bladder cancer to run in families. A family history of hereditary nonpolyposis colorectal cancer, also called Lynch syndrome, can increase your risk of cancer in your urinary system, as well as in your colon, uterus, ovaries and other organs.
The World Health Organization recognizes a papilloma as a papillary tumor with a fine fibrovascular stalk supporting an epithelial layer of transitional cells with normal thickness and cytology (Epstein et al, 1998). Papillomas are a rare benign condition usually occurring in younger patients.
C. Transitional Cell Carcinoma
Approximately 90% of all bladder cancers are transitional cell carcinomas. These tumors most commonly appear as papillary, exophytic lesions (Figure 20-2B); less commonly, they may be sessile or ulcerated. Whereas the former group are usually superficial in nature, sessile growths are often invasive. The World Health Organization has proposed that transitional cell carcinomas be divided into 3 categories on the basis of urothelial architecture, cell size, nuclear size and shape, the presence or absence of nucleoli, hyperchromatism, and the number of mitoses present (Epstein et al, 1998).
Carcinoma in situ (CIS) is recognizable as flat, anaplastic epithelium. The urothelium lacks the normal cellular polarity, and cells contain large, irregular hyperchromatic nuclei with prominent nucleoli (Figure 20-2C). Carcinoma in situ may occur either close to or remote from an exophytic lesion or, rarely, it may occur as focal or diffuse lesions in a patient without macroscopic tumors. It has a variable natural history, but many cases progress to invasive disease. In addition, exophytic lesions occurring with CIS are more likely to recur and invade than those without CIS. 
The frequency of tumor invasion, recurrence, and progression is strongly correlated with tumor grade (Lutzeyer, Rubben, and Dahm, 1982; Torti et al, 1987): Progression is noted infrequently in patients with papillary urothelial neoplasm of low malignant potential, in less than 5% of patients with low-grade papillary urothelial carcinoma, and in 15-40% of patients with high-grade papillary urothelial carcinoma (Epstein et al, 1998).
D. Nontransitional Cell Carcinomas
1. Adenocarcinoma - Adenocarcinomas account for less than 2% of all bladder cancers. Primary adenocarcinomas of the bladder may be preceded by cystitis and metaplasia. Histologically, adenocarcinomas are mucus-secreting and may have glandular, colloid, or signet-ring patterns. Whereas primary adenocarcinomas often arise along the floor of the bladder, adenocarcinomas arising from the urachus occur at the dome. Both tumor types are often localized at the time of diagnosis, but muscle invasion is usually present. Five-year survival is usually less than 40%, despite aggressive surgical management (Kramer et al, 1979; Malek, Rosen, and O'Dea, 1983; Abenoza, Manivel, and Fraley, 1987; Bernstein et al, 1988).
2. Squamous cell carcinoma - Squamous cell carcinoma accounts for between 5% and 10% of all bladder cancers in the United States and is often associated here with a history of chronic infection, vesical calculi, or chronic catheter use. It may also be associated with bilharzial infection owing to Schistosoma haematobium, because squamous cell carcinoma accounts for approximately 60% of all bladder cancers in Egypt, parts of Africa, and the Middle East, where this infection is prevalent (El-Bolkainy et al, 1981). These tumors are often nodular and invasive at the time of diagnosis. Histologically they appear as poorly differentiated neoplasms composed of polygonal cells with characteristic intercellular bridges. Keratinizing epithelium is present, although often in small amounts.
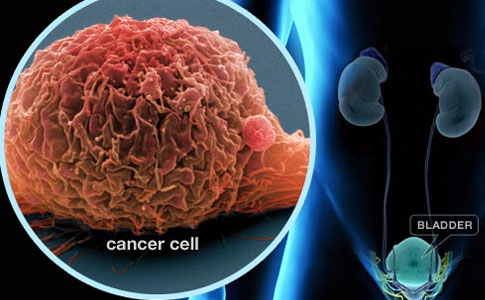
Bladder cancer is the sixth most common cancer in the United States. For the year 2014 it is estimated that more than 74,000 Americans will be diagnosed with bladder cancer and more than 15,000 will die of the disease. In recent decades there has been a steady increase in the incidence of bladder cancer. However, doctors are making progress in treatment, and survival rates are improving. But what are its symptoms? How should it be treated? The following information should help you talk to an urologist about this condition.
What happens under normal conditions?
The bladder is a hollow balloon-shaped mostly muscular organ that stores urine until ready for release. The urine is produced in the kidneys. It flows through tubes called the ureters into the bladder and is discharged through the urethra during urination. The bladder muscle aids urination by contracting (tightening) to help force out the urine.
A thin surface layer called the urothelium lines the inside of the bladder. Next is a layer of loose connective tissue called the lamina propria . Covering the lamina propria is the bladder muscle. Outside of the bladder is a layer of fat.
What causes bladder cancer?
The ways in which bladder cancers develop and progress are only partly understood. However, a number of substances that cause the cancers to develop have been identified. Chief among them are cancer-causing agents in cigarette smoke and various industrial chemicals. Cigarette smoking alone has been estimated to cause 50 percent of all bladder cancer cases in the United States. Long-term workplace exposure to chemical compounds such as paints and solvents has been estimated to cause another 20 to 25 percent of bladder cancer cases. Carcinogens in the blood stream are filtered out by the kidneys to eliminate them from the body. However, these carcinogens remain in the bladder for a few hours interacting with the lining of the bladder before they are removed by urination. Through this process the bladder becomes a high risk organ for cancer, particularly in smokers.
More than 90 percent of all bladder cancers originate in the urothelium , the inner lining of the bladder. The majority of diagnosed bladder tumors are confined to the urothelium or the lamina propria and have not invaded the bladder muscle.
3. Undifferentiated carcinomas - Undifferentiated bladder carcinomas, which are rare (accounting for less than 2%), have no mature epithelial elements. A small-cell type has been described that histologically resembles similar lesions of the lung (Mills et al, 1987).
4. Mixed carcinoma - Mixed carcinomas constitute 4-6% of all bladder cancers and are composed of a combination of transitional, glandular, squamous, or undifferentiated patterns. The most common type comprises transitional and squamous cell elements (Murphy, 1989). Most mixed carcinomas are large and infiltrating at the time of diagnosis.
E. Rare Epithelial and Nonepithelial Cancers
Rare epithelial carcinomas identified in the bladder include villous adenomas, carcinoid tumors, carcinosarcomas, and melanomas. Rare nonepithelial cancers of the urinary bladder include pheochromocytomas, lymphomas, choriocarcinomas, and various mesenchymal tumors (hemangioma, osteogenic sarcoma, and myosarcoma) (Murphy, 1989). Cancers of the prostate, cervix, and rectum may involve the bladder by direct extension. The most common tumors metastatic to the bladder include (in order of incidence) melanoma, lymphoma, stomach, breast, kidney, and lung (Murphy, 1989; Goldstein, 1967).
Clinical Findings
Hematuria is the presenting symptom in 85-90% of patients with bladder cancer. It may be gross or microscopic, intermittent rather than constant. In a smaller percentage of patients, it is accompanied by symptoms of vesical irritability: frequency, urgency, and dysuria. Irritative voiding symptoms seem to be more common in patients with diffuse CIS (Farrow et al, 1977). Symptoms of advanced disease include bone pain from bone metastases or flank pain from retroperitoneal metastases or ureteral obstruction.
Most patients with bladder cancer have no pertinent physical signs, owing to the superficial nature of their disease. However, patients with large-volume or invasive tumors may be found to have bladder wall thickening or a palpable mass - findings that may be detected on a careful bimanual examination under anesthesia. Masses palpable before transurethral resection but not identifiable thereafter are most often either very large superficial lesions or tumors that have penetrated the more superficial layers of the bladder wall (ie, T2). Those that remain palpable are often locally extensive (≥ T3a). Tumors that are movable anteroposteriorly are classified as a lower stage than T3b, whereas those that are fixed or are invading contiguous organs (rectum, prostate, vagina) are considered to be stage T4.
Hepatomegaly and supraclavicular lymphadenopathy are signs of metastatic disease. Lymphedema from occlusive pelvic lymphadenopathy may be seen occasionally.
1. Routine testing - The most common laboratory abnormality is hematuria. It may be accompanied by pyuria, which on occasion may result from concomitant urinary tract infection. Azotemia may be noted in patients with ureteral occlusion owing to the primary bladder tumor or lymphadenopathy. Anemia may be a presenting symptom owing to chronic blood loss, or replacement of the bone marrow with metastatic disease.
2. Urinary cytology - Exfoliated cells from both normal and neoplastic urothelium can be readily identified in voided urine. Larger quantities of cells can be obtained by gently irrigating the bladder with isotonic saline solution through a catheter or cystoscope. Examination of cytologic specimens obtained in either fashion allows for tumor detection either at the time of initial presentation or during follow-up. Cytologic examination of exfoliated cells may be especially useful in screening high-risk populations and assessing response to treatment. Detection rates vary depending on the adequacy of the specimen and the grade and volume of the tumor.
Cytologic preparations are made by fixing exfoliated cells, placing them on glass slides, and staining them. High-grade and infiltrating carcinomas are commonly detected, but low-grade or superficial carcinomas may be missed.
3. Other markers - The sensitivity of voided urine cytologic tests depends on a variety of factors, including the number of urine specimens examined, the stage and grade of the bladder tumor, and the expertise of the cytopathologist. Consequently, newer tests that are also performed on voided urine specimens are currently being developed and validated to determine their ability to reliably predict the presence of bladder cancer. These tests include, but are not limited to, the BTA test (Bard Urological, Covington, GA), the BTA stat test (Bard Diagnostic Sciences, Inc, Redmond, WA), the BTA TRAK assay (Bard Diagnostic Sciences, Inc), determination of urinary nuclear matrix protein (NMP22; Matritech Inc, Newton, MA), quantification of urinary fibrinogen/fibrin degradation products (AuraTek FDP; PerImmune Inc, Rockville, MD), identification of the Lewis X antigen on exfoliated urothelial cells, and the determination of telomerase activity in exfoliated cells. Several studies have examined the performance of these voided urinary markers for the detection and follow-up of patients with bladder cancer (summarized in Grossfeld and Carroll, 1998; Grossfeld et al, 2001; Konety and Getzenberg, 2001) (Table 20-1).
Most of the studies performed to date have evaluated these voided markers in patients with known bladder cancer, either primary or recurrent, and have compared the results with those obtained in a control group without bladder cancer. Although these tests may, in many clinical situations, complement traditional evaluation and surveillance techniques, more information on their performance is needed before they are performed routinely. Such exfoliated markers, if they prove to be both specific and sensitive, may play an important role in the initial evaluation and follow-up of patients with bladder cancer.
In addition to enhancing the detection of bladder cancer, such tests may give important information on the natural history of the bladder cancer detected. For example, loss of blood group antigens may correlate with bladder cancer stage and risk of progression. Fradet and colleagues (1990) identified a set of bladder carcinoma cell antigens detected by monoclonal antibodies whose expression correlates not only with tumor stage but also with progression rates.
Although bladder cancers may be detected by various imaging techniques, their presence is confirmed by cystoscopy and biopsy. Imaging is therefore used to evaluate the upper urinary tract and, when infiltrating bladder tumors are detected, to assess the depth of muscle wall infiltration and the presence of regional or distant metastases (See and Fuller, 1992). Intravenous urography remains one of the most common imaging tests for the evaluation of hematuria. Bladder tumors may be recognized as pedunculated, radiolucent filling defects projecting into the lumen (Figure 20-3); nonpapillary, infiltrating tumors may result in fixation or flattening of the bladder wall. Hydronephrosis from ureteral obstruction is usually associated with deeply infiltrating lesions and poor outcome after treatment (Haleblian et al, 1998).
Superficial (Ta, Tis) bladder cancers staged with a properly performed transurethral resection and examination under anesthesia do not require additional imaging of the bladder or pelvic organs. However, higher-stage lesions are often understaged, and the addition of imaging may be useful. Both computed tomography and magnetic resonance imaging (Figure 20-4) have been used to characterize the extent of bladder wall invasion and detect enlarged pelvic lymph nodes, with overall staging accuracy ranging from 40% to 85% for computed tomography and from 50% to 90% for magnetic resonance imaging (Amendola et al, 1986; Koss et al, 1981; Fisher, Hricak, and Tanagho, 1985; Husband et al, 1989; Wood et al, 1988). Both techniques rely on size criteria for the detection of lymphadenopathy: Lymph nodes larger than 1 cm are thought to be suggestive of metastases; unfortunately, small-volume pelvic lymph node metastases are often missed. Because invasive bladder cancers may metastasize to the lung or bones, staging of advanced lesions is completed with chest x-ray and radionuclide bone scan.
E. Cystourethroscopy and Tumor Resection
The diagnosis and initial staging of bladder cancer is made by cystoscopy and transurethral resection. Cystoscopy can be done with either flexible or rigid instruments, although the former may be associated with less discomfort. For either, topical anesthetic solutions are injected directly into the urethra. Superficial, low-grade tumors usually appear as single or multiple papillary lesions; most measure less than 3 cm in diameter. Higher-grade lesions are larger and sessile. Carcinoma in situ may appear as flat areas of erythema and mucosal irregularity.
Once a tumor is visualized or suspected, the patient is scheduled for examination under anesthesia and transurethral resection or biopsy of the suspicious lesion. The objectives are tumor diagnosis, assessment of the degree of bladder wall invasion (staging), and complete excision of the low-stage lesions amenable to such treatment.
Patients are placed in the lithotomy position. A careful bimanual examination is performed. The presence, position, and size of any masses are noted, along with any degree of fixation to contiguous structures. Cystoscopy is repeated with a lens that permits complete visualization of the entire bladder surface. A resectoscope is then placed into the bladder, and visible tumors are removed by electrocautery. Suspicious areas may have biopsies done with cup biopsy forceps and the areas may be cauterized with an electrode. Some clinicians routinely perform random bladder biopsies of normal-appearing urothelium both close to and remote from the tumor. Although detection of dysplasia or CIS occurs in up to 65% of selected patients, probably less than 15% of all patients with initial bladder tumors have such lesions (Althausen, Prout, and Dal, 1976; Wolf and Hojgaard, 1983). Those who do are at a greater risk of both recurrence and progression to a higher stage.
Natural History & Selection of Treatment
A. Standard Histopathological Assessment
The natural history of bladder cancers is defined by 2 separate but related processes: tumor recurrence and progression. Progression, including metastasis, represents the greater biologic risk. However, recurrence, even without progression, represents substantial patient morbidity in that it requires periodic reevaluation (cytology, cystoscopy, etc), repeat endoscopic ablation, and often intravesical chemotherapy (which may be costly, uncomfortable, and associated with complications). Treatment decisions are made after tumor staging has been completed. Such decisions are based variously on tumor stage (TNM), grade, size, multiplicity, and recurrence pattern (Table 20-2).
At initial presentation, approximately 50-70% of bladder tumors are superficial - stage Tis or Ta (Cutler, Heney, and Friedell, 1982). Invasion into the lamina propria or muscle wall is identified in a smaller number of patients, approximately 28% and 24%, respectively; regional or distant metastases are found in approximately 15%. Unfortunately, 80% of patients with invasive or metastatic disease have no previous history of bladder cancer (Brawn, 1982; Kaye and Lange, 1982). Bladder carcinomas also may be stratified at the time of initial presentation on the basis of grade: Approximately 43% of tumors are classified as grade I; 25% as grade II; and 32% as grade III (Gilbert et al, 1978). There are strong correlations between tumor grade and stage and tumor recurrence, progression, and survival (Frazier et al, 1993). Patients with low-stage, low-grade disease have a low risk (< 5%) of progression to invasive disease, while as many as 40% of patients with low-stage but high-grade disease will progress with extended follow-up (Herr, 2000). Disease-free survival is excellent for patients with superficial disease (PO, PI, PIS, 80-88%). However, it falls for patients with P2 (53-80%), P3 (39-68%), and P4 (25-40%) tumors (Stein et al, 2001; Frazier et al, 1993; Thrasher et al, 1994) - owing to the greater likelihood of metastasis in tumors of higher stage. Whereas lymph node metastases are uncommon (5%) in tumors of low stage, they are increasingly more common in higher-stage tumors: 10-30% for P3A, 31-46% for P3B, and 35-64% for P4 (Stein et al, 2001; Frazier et al, 1993). In patients with organ-confined disease, the presence of pelvic lymph node metastases appears to be the most important prognostic factor (Vieweg et al, 1999).
Although metastasis is less common with superficial bladder cancers, such tumors may progress; most recur and require additional treatment. Stratification of superficial disease into different grades and stages (Ta and T1) is of clinical benefit, because progression and recurrence are related to both. Tumor progression occurs in less than 6% of patients with Ta disease, but in up to 53% of those with T1 disease, with or without concomitant CIS (Heney et al, 1983; Pauwels et al, 1988; Abel et al, 1988; Cookson et al, 1997). It occurs in 10-20% of patients with grade I tumors, 19-37% with grade II tumors, and 33-64% with grade III tumors (Torti et al, 1987; Lutzeyer, Rubben, and Dahm, 1982).
What are the risk factors for bladder cancer?
Smoking: Smoking is the greatest risk factor. Smokers get bladder cancer twice as often as people who don't smoke.
Chemical Exposure: Some chemicals used in the making of dye have been linked to bladder cancer. People who work with chemicals called aromatic amines may have higher risk. These chemicals are used in making rubber, leather, printing materials, textiles and paint products.
Race: Caucasians are twice as likely to develop bladder cancer as are African Americans or Hispanics. Asians have the lowest rate of bladder cancer.
Age: The risk of bladder cancer increases as you get older.
Gender: While men get bladder cancer more often than women, recent statistics show an increase in the number of women being diagnosed with the disease. Unfortunately, because the symptoms of bladder cancer are similar to those of other gynecologic and urinary diseases affecting women, women may be diagnosed when their disease is at a more advanced stage.
Chronic bladder inflammation: Urinary infections, kidney stones and bladder stones don't cause bladder cancer, but they have been linked to it.
Personal history of bladder cancer: People who have had bladder cancer have a higher chance of getting another tumor in their urinary system. People whose family members have had bladder cancer may also have a higher risk.
Birth defects of the bladder: Very rarely, a connection between the belly button and the bladder doesn't disappear as it should before birth and can become cancerous.
Arsenic: Arsenic in drinking water has been linked to a higher risk of bladder cancer.
Earlier Treatment: Some drugs (in particular Cytoxan/cyclophosphamide) or radiation used to treat other cancers can increase the risk of bladder cancer.
Some drugs (in particular Cytoxan/cyclophosphamide) or radiation used to treat other cancers can increase the risk of bladder cancer.
What are the signs and symptoms of bladder cancer?
The most common clinical sign of bladder cancer is painless gross hematuria, blood in the urine that can easily be seen. Two features that tend to mask the severity of the gross hematuria and may influence patients to postpone seeking immediate medical care are 1) the bleeding may be occasional and short-lived; and 2) there is likely to be no pain associated with the bleeding. In addition, it may be that the tumors do not produce enough blood for a patient to see (microscopic hematuria) and are only detected with the help of special chemicals and/or a microscope after a urine test is done by a physician.
However, blood in the urine does not necessarily mean a diagnosis of bladder cancer. Infections, kidney stones as well as aspirin and other blood-thinning medications may cause bleeding. In fact, the overwhelming majority of patients who have microscopic hematuria do not have cancer.
Irritation when urinating, urgency, frequency and a constant need to urinate may be symptoms a bladder cancer patient initially experiences. Oftentimes, though, these are merely symptoms of a urinary tract infection and antibiotics become the first line of treatment. To make the necessary distinction between an infection and something more serious, it is critical that a urinalysis and/or culture are done to detect any bacteria in the urine. If the culture is negative for bacteria, patients should be referred to a urologist for further testing.
Tumor recurrence is related to history of disease and grade, number, and size of the tumor. It is more common in the first 12 months after diagnosis (but can become manifest many years later), and patients with one recurrence are more likely to have another. Patients with T1, multiple (> 4), large (> 5), or high-grade tumors are at greater risk, as are those with either CIS or severe dysplasia in normal-appearing urothelium remote from the tumor site (Heney et al, 1983; Wolf, Olsen, and Hojgaard, 1985).
Conventional histopathologic analysis of bladder tumors, including determination of tumor grade and stage, may not reliably predict the behavior of many bladder cancers. Assessment of molecular markers of disease, most often with immunohistochemical methods, may complement traditional assessment of cancer stage and grade and better predict outcome. Some markers may be assessed in biopsy specimens, whereas others may be prognostic only when evaluating cystectomy specimens.
Tumor growth and metastasis require the growth of new blood vessels, a process called angiogenesis. New vessel growth is tightly regulated by both angiogenic stimulators, such as the fibroblastic growth factors and vascular endothelial growth factor, and by angiogenic inhibitors, such as thrombospondin-1 and angiostatin. Immunohistochemical methods have been developed for quantification of angiogenesis in a given tumor by measuring microvessel density. Microvessel density is a useful prognostic indicator for a variety of human malignancies, including bladder cancer. In bladder cancer, microvessel density has been associated with lymph node metastases, disease progression, and overall survival in patients with invasive bladder cancer treated with radical cystectomy (Dickinson et al, 1994; Jaeger et al, 1995; Bochner et al, 1997). Furthermore, immunohistochemical expression of thrombospondin-1 has been reported to be significantly associated with both tumor recurrence and overall survival (Grossfeld et al, 1997).
Normal cellular proliferation is the result of an orderly progression through the cell cycle, whereas malignancy is characterized by uncontrolled cell growth. Cell cycle-associated protein complexes composed of cyclins and cyclin-dependent kinases tightly regulate this progression. These protein complexes phosphorylate key proteins involved in cell cycle transition points, including the protein encoded by the retinoblastoma (pRB) and p53 genes. Loss of cell cycle control may be an early step in the development of carcinogenesis. The most extensively characterized molecular marker in patients with invasive bladder cancer is p53 expression. The p53 gene is a tumor suppressor gene that plays a key role in the regulation of the cell cycle. When DNA damage occurs, the level of p53 protein increases, causing cell cycle arrest and repair of DNA. Mutations in the p53 gene result in the production of an abnormal protein product, allowing cells with damaged DNA to continue through the cell cycle. The altered p53 protein has a prolonged half-life compared with the wild-type protein, allowing for its detection by immunohistochemical techniques. Increased p53 immunoreactivity has been found in high-grade and high-stage bladder cancers, and it is associated with disease progression and decreased overall and disease-specific survival. Patients with altered p53 expression (indicating possible mutation of the p53 gene) appear to have an increased risk for disease recurrence and a decreased overall survival when compared with patients with normal p53 expression (Esrig et al, 1995). Cancers that are p53 positive are associated with recurrence rates of 62% for pT1, 56% for pT2, and 80% for P3a, compared with 7%, 12%, and 11%, respectively, for cancers without p53 reactivity. This information has recently led to a clinical trial in which patients are assigned to adjuvant treatment after radical cystectomy based on the p53 status of their tumor. Assessment of p21, another cell cycle protein, may complement knowledge gained from assessment of p53 expression (Stein et al, 1998).
The retinoblastoma (Rb) gene, a tumor suppressor gene, is expressed in several human tumors, including transitional cell carcinoma of the bladder. Rb alteration, as determined by immunohistochemical methods, is associated with high-grade, high-stage bladder cancers. In addition, Rb alteration appears to be significantly associated with decreased overall survival in such patients (Cordon-Cardo et al, 1992; Logothetis et al, 1992). Studies in which both p53 and Rb have been examined in patients with invasive bladder cancer suggest that bladder tumors with alterations in both genes have a poorer prognosis and decreased overall survival when compared with tumors with wild-type p53 and Rb. Tumors with an alteration of only one of these genes behave in an intermediate fashion.
Assessment of other markers that may correlate with outcome in patients with bladder cancer includes that of tumor growth fraction (proliferative index) and cellular adhesion molecule expression (E-cadherin) (Okamura et al, 1990; Lipponen and Eskelinen, 1995).
Patients with superficial bladder cancers can be treated with transurethral resection followed by selective intravesical chemotherapy or immunotherapy. Patients with initial low-grade, small tumors are at low risk of progression and may be treated by transurethral resection alone followed by surveillance. Patients with T1, high-grade, multiple, large, recurrent tumors or those associated with CIS in remote bladder biopsies are at a higher risk of progression and recurrence and should be considered candidates for intravesical chemotherapy or immunotherapy after complete and careful transurethral resection. Management of T1 tumors is somewhat controversial; some clinicians advise radical cystectomy, especially for grade III lesions, which are associated with a high rate of progression. However, progression rates can be reduced by intravesical chemotherapy (Herr et al, 1989; Cookson and Sarosdy, 1992). Recurrence of T1 disease after a trial of intravesical therapy warrants more aggressive therapy (Herr, 1991; Herr and Sogani, 2001).
Patients with more invasive, but still localized, tumors (T2, T3) are candidates for more aggressive local treatment, including partial or radical cystectomy, or a combination of either irradiation or surgery and systemic chemotherapy. It has been suggested that selected patients with stage T2 disease may be treated adequately with transurethral resection alone. However, this mode of treatment leaves residual disease in most patients (Henry et al, 1988; Herr, 1987; Solsona et al, 1998). Superficial ductal or acinar in situ carcinoma of the prostatic urethra, not invading the basement membrane or prostatic stroma, may be treated with transurethral resection and intravesical chemotherapy or immunotherapy rather than cystectomy (Bretton et al, 1989). However, patients with more extensive involvement of the prostatic urethra by transitional cell carcinoma, or recurrence after conservative therapy, require more aggressive therapy (Schellhammer, Bean, and Whitmore, 1977). Patients with unresectable local tumors (T4B) are candidates for systemic chemotherapy, followed by surgery (or possibly irradiation). Patients with either local or distant metastases should receive systemic chemotherapy followed by the selective use of either irradiation or surgery, depending on the response.
Treatment
In most patients with superficial bladder cancer, tumors recur. Because a certain percentage of these are of higher stage or grade, attempts to reduce recurrence should be undertaken.
Immunotherapeutic or chemotherapeutic agents can be instilled into the bladder directly via catheter, thereby avoiding the morbidity of systemic administration in most cases. Intravesical therapy can have a prophylactic or therapeutic objective, either to reduce recurrence in patients whose tumors have been completely resected or to eradicate existing disease in patients whose superficial tumors are so extensive that complete transurethral resection is not possible. Therefore, intravesical chemotherapy or immunotherapy may be delivered in 3 different fashions to achieve individual goals (Table 20-3). Considerable experience has been gained, but comparison of different agents is difficult owing to the paucity of randomized trials and variations in dose, contact time, patient population, and intervals between treatments. Most agents are administered weekly for 6 weeks. Maintenance therapy (ie, monthly or bimonthly intravesical therapy) may decrease recurrence rates further. Although local toxicity is relatively common - primarily irritative voiding symptoms - systemic toxicity is rare because of the limited absorption of drugs across the lumen of the bladder. Severe systemic complications can be avoided by not administering intravesical chemotherapy in patients with gross hematuria. Efficacy may be improved by increasing contact time and drug concentration (ie, by restricting fluid intake before administration, asking the patient to lie in different positions during treatment, avoiding instillation of air during drug administration, and requiring the patient to avoid urinating for 1-2 h thereafter). The most common agents in the United States are mitomycin C, thiotepa, doxorubicin, and bacillus Calmette-Guerin. Patients in whom treatment with one agent fails may respond to another.
1. Mitomycin C - Mitomycin C is an antitumor, antibiotic, alkylating agent that inhibits DNA synthesis. With a molecular weight of 329, systemic absorption is minimal. Between 39% and 78% of patients with residual tumor experience a complete response to intravesical mitomycin C (Kowalkowski and Lamm, 1988), and recurrence is reduced in 2-33% after complete transurethral resection (Herr, Laudone, and Whitmore, 1987). Side effects are noted in 10-43% of patients and consist largely of irritative voiding symptoms including urinary frequency, urgency, and dysuria (Soloway, 1989). Unique to this drug is the appearance of a rash on the palms and genitalia in approximately 6% of patients, but this effect can be alleviated if patients wash their hands and genitalia at the time of voiding after intravesical administration.
2. Thiotepa - Thiotepa is an alkylating agent with a molecular weight of 189. Although various doses have been used, 30 mg weekly seems to be sufficient. Up to 55% of patients respond completely. Most series show significantly lower recurrence rates in patients taking thiotepa than in those taking a placebo (Herr, Laudone, and Whitmore, 1987; Kowalkowski and Lamm, 1988; Prout et al, 1983). Cystitis is not uncommon after instillation but is usually mild and self-limited. Myelosuppression manifested as leukopenia and thrombocytopenia occurs in up to 9% of patients owing to systemic absorption. A complete blood count should be obtained in all patients before successive instillations.
3. Doxorubicin - Doxorubicin is an intercalating agent of high molecular weight (580). Complete response rates vary among series, with a mean of 38% (Kowalkowski and Lamm, 1988). As a prophylactic agent, the benefit of intravesical administration of this drug ranges from 10% to 23% (Herr, Laudone, and Whitmore, 1987). Cystitis is not uncommon, but systemic toxicity is rare.
4. Bacillus Calmette-Guerin (BCG) - Bacillus Calmette-Guerin (BCG) is an attenuated strain of Mycobacterium bovis. Many different strains of BCG exist, and the marketed preparations vary in the number, pathogenicity, viability, and immunogenicity of organisms (Catalona and Ratliff, 1990). The exact mechanism by which BCG exerts its antitumor effect is unknown, but it seems to be immunologically mediated. Mucosal ulceration and granuloma formation are commonly seen after intravesical instillation. Activated helper T lymphocytes can be identified in the granulomas, and interleukin-2 reportedly can be detected in the urine of treated patients (Haaf, Catalona, and Ratliff, 1986). Bacillus Calmette-Guerin has been shown to be very effective both therapeutically and prophylactically. It appears to be the most efficacious intravesical agent for the management of CIS. Complete responses are recorded in 36-71% of patients with residual carcinoma (Herr, Laudone, and Whitmore, 1987; Catalona and Ratliff, 1990). Recurrence rates are reduced substantially in patients treated after endoscopic resection (11-27% versus a 70% recurrence after endoscopic resection alone) (Catalona and Ratliff, 1990; Herr, Laudone, Whitmore, 1987; Camacho et al, 1980; Herr et al, 1985; Lamm, 1985). Bacillus Calmette-Guerin has been shown to be superior to intravesical chemotherapy in preventing recurrence in patients with high-risk superficial bladder cancer (Lamm et al, 1991). Although BCG appears to be effective in delaying progression of high-risk superficial bladder cancer, 40-50% of these patients will experience disease progression with extended follow-up and many patients will ultimately require cystectomy (Cookson et al, 1997; Herr et al, 1995; Davis et al, 2002). The optimal schedule of intravesical BCG is not known with certainty; the most commonly recommended induction regimen is BCG weekly for 6 weeks followed by a period of 6 weeks where no BCG is given. At 12 weeks, if no cancer is identified, BCG is given weekly for 3 weeks. Continuous administration of BCG for more than 6 weeks may be immunosuppressive. Maintenance therapy at 3- to 6-month intervals should be considered in high-risk patients (Lamm et al, 2000). Combining dermal scarification with intravesical instillation appears to result in no enhanced benefit (Badalament et al, 1987; Hudson et al, 1987). Side effects of intravesical BCG administration are relatively common, although severe complications are uncommon. Most patients experience some degree of urinary frequency and urgency. Hemorrhagic cystitis occurs in approximately 7% of patients, and evidence of distant infection is found in less than 2%. Patients with mild systemic or moderate local symptoms should be treated with isoniazid (300 mg daily), and the dosage of BCG should be reduced. Isoniazid is continued while symptoms persist and restarted 1 day before the next instillation.
Patients with severe systemic symptoms should have instillations stopped. Patients with prolonged high fever (> 103 °F), symptomatic granulomatous prostatitis, or evidence of systemic infection require treatment with isoniazid and rifampin (600 mg daily). Patients with signs and symptoms of BCG sepsis (eg, high fever, chills, confusion, hypotension, respiratory failure, jaundice) should be treated with isoniazid, rifampin, and ethambutol (1200 mg). The addition of cycloserine (500 mg twice daily) or prednisolone (40 mg daily) increases survival rates (Lamm, 1992).
5. New intravesical agents and approaches - The rate of metachronous tumor recurrence is high compared with that of low-grade cancers occurring in other organs (eg, nasopharynx, colon). Recurrence of superficial bladder cancer is related to cancer stage, grade and number of tumors, associated dysplasia, and DNA content. Recurrent tumors may be due to regrowth of previously resected cancers, growth of new cancers at remote sites, or implantation and subsequent proliferation of cells released into the bladder at the time of endoscopic treatment of the original tumor. Several investigators have studied the efficacy of single-dose therapy delivered at the time of complete transurethral resection (Tolley et al, 1988; Oosterlinck et al, 1993; Rajala et al, 1999). Such therapy has been shown to decrease recurrence rates, probably by decreasing the risk of tumor cell implantation at the time of initial cancer resection. Trials of interferon-alpha, bropirimine (an oral immunomodulator), and valrubicin (an anthracycline derivative) suggest that these agents, either alone or perhaps in combination with other agents, may be effective in either high-risk patients or those who fail to respond to first-line therapy (Belldegrun et al, 1998; Sarosdy et al, 1998; Steinberg et al, 2000). Preliminary studies suggest that low-dose BCG, in combination with interferon, may be successful in 50-60% of patients with high-risk superficial bladder cancer who have failed BCG therapy alone (O'Donnell, Krohn, and DeWolf, 2001). Given the relatively easy accessibility of bladder cancer cells to direct application of therapeutic agents, such cancers may be ideal targets for genetic manipulation (Miyake et al, 1998).
1. Transurethral resection - Transurethral resection is the initial form of treatment for all bladder cancers. It allows a reasonably accurate estimate of tumor stage and grade and the need for additional treatment. Patients with single, low-grade, noninvasive tumors may be treated with transurethral resection alone; those with superficial disease but high-risk features should be treated with transurethral resection followed by selective use of intravesical therapy, as described above. Transurethral resection alone has rarely been used in the management of patients with invasive bladder cancer because of a high likelihood of recurrence and progression. Such an approach has been used infrequently for carefully selected patients with comorbid medical conditions and either no residual disease or minimal disease only at restaging transurethral resection of bladder tumor (Herr, 1987; Solsona et al, 1998). Careful follow-up of patients with superficial bladder cancers is mandatory because disease will recur in 30-80% of patients, depending on cancer grade, tumor stage, and number of tumors. Disease status at 3 months after initial resection is an important predictor of the risk of subsequent recurrence and progression (Holmang and Johansson, 2002; Solsona et al, 2000). All patients who undergo transurethral resection should undergo cystoscopy at 3 months. For patients who presented initially with solitary, low-grade lesions and who are free of recurrence at 3 months, repeat cystoscopy at 1 year is suggested. Patients who presented initially with multiple or higher-grade lesions (or both) and those who have recurrences at 3 months require more careful surveillance. In such patients, cystoscopy at 3-month intervals is necessary. Although periodic cystoscopy is suggested for all patients with a history of bladder cancer, the risk of recurrence decreases as the tumor-free interval increases. After 5 years without recurrence, the risk of recurrence has been estimated to be 22%; the rate is 2% for 10 years (Morris et al, 1995).
2. Partial cystectomy - Patients with solitary, infiltrating tumors (T1-T3) localized along the posterior lateral wall or dome of the bladder are candidates for partial cystectomy, as are patients with cancers in a diverticulum. Disease remote from the primary tumor must be excluded by random bladder biopsies preoperatively. Few patients with invasive transitional cell carcinomas are candidates (Utz et al, 1973). To minimize tumor implantation resulting from contamination of the wound with cancer cells at the time of surgery, short-course, limited-dose (1000-1600 cGy) irradiation can be used, and an intravesical chemotherapeutic agent can be instilled preoperatively (Ojeda and Johnson, 1983; Van der Werf-Messing, 1969). Although survival rates of well-selected patients may approach those for patients with similar-stage tumors treated by radical cystectomy, local recurrences are common (Whitmore, 1983; Cummings et al, 1978; Merrell, Brown, and Rose, 1979; Schoborg, Sapolsky, and Lewis, 1979; Sweeney et al, 1992). Given current techniques of bladder replacement surgery, partial cystectomy is rarely indicated in the management of patients with invasive bladder cancer.
3. Radical cystectomy - Radical cystectomy implies removal of the anterior pelvic organs: in men, the bladder with its surrounding fat and peritoneal attachments, the prostate, and the seminal vesicles; in women, the bladder and surrounding fat and peritoneal attachments, cervix, uterus, anterior vaginal vault, urethra, and ovaries. This remains the "gold standard" of treatment for patients with muscle invasive bladder cancer. Disease-free survival 5 years after surgery is based on tumor stage: 88% for patients with P0, Pa, or PIS disease; 80% for patients with P1 disease; 81% for patients with P2 disease; 68% for patients with P3a and 47% for those with P3b disease; and 44% for patients with P4a disease (Stein et al, 2001). Recurrences after surgery usually occur within the first 3 years. Local pelvic recurrence rates are low (7-10%); most patients who fail therapy have distant disease recurrence.
The risk of urethral tumor occurrence or recurrence in men who undergo radical cystectomy is 6.1-10.6%. Risk factors for urethral tumor involvement in men include infiltration of the prostatic stroma or prostatic urethra with cancer or CIS. Patients with these risk factors are candidates for urethrectomy either at the time of radical cystectomy or as a separate procedure (Zabbo and Montie, 1984; Schellhammer and Whitmore, 1976). Although prostatic urethral disease is a risk factor for urethral recurrence, recent evidence suggests that urethrectomy may be omitted and orthotopic urinary diversion performed safely in men with only proximal prostatic urethral involvement and a negative urethral margin at radical cystectomy (Iselin et al, 1997).
Urethrectomy was once routinely performed in all women undergoing radical cystectomy. However, recent clinical experience suggests that bladder replacement may be an acceptable procedure in women as well as men. Women with bladder cancer who have a clear urethral margin at the time of cystectomy are candidates for this procedure. Approximately 66% of women undergoing radical cystectomy for the management of bladder cancer fall into this group (Stein et al, 1995; Stenzl et al, 1995; Stein et al, 1998).
A bilateral pelvic lymph node dissection is usually performed simultaneously with radical cystectomy. Lymph node metastases are identified in approximately 20-35% of patients (Lieskovsky, Aherling, and Skinner, 1988; Stein et al, 2001) - an incidence that reflects the inability of any imaging mode to identify consistently small-volume lymph node metastases preoperatively. Patients with lymph node metastases have a poorer prognosis. However, some patients (10-33%) with limited disease in regional lymph nodes may be cured by radical cystectomy and lymphadenectomy (Lerner et al, 1993; Vieweg et al, 1999; Stein et al, 2001). Patients with fewer than 5 positive lymph nodes and organ-confined disease in the primary tumor tend to have a better prognosis than patients with more extensive disease. These patients may also benefit from adjuvant chemotherapy (see section D, below).
Urinary diversion may be accomplished using a variety of techniques. Methods have been developed that allow construction of reservoirs that are continent and do not require the patient to wear an external appliance for collection of urine.
External beam irradiation (5000-7000 cGy), delivered in fractions over a 5- to 8-week period, is an alternative to radical cystectomy in patients with deeply infiltrating bladder cancers. Treatment is generally well tolerated, but approximately 15% of patients may have significant bowel, bladder, or rectal complications. Five-year survival rates for stages T2 and T3 disease range from 18% to 41% (Timmer, Hartliff, and Hooijkaas, 1985; Goffinet et al, 1975; Corcoran et al, 1985; Miller, 1977; Woon et al, 1985; Quilty and Duncan, 1986; Rider and Evans, 1976; Cummings et al, 1976). Unfortunately, local recurrence is common, occurring in approximately 33-68% of patients. Consequently, radiation as monotherapy is usually offered only to those patients who are poor surgical candidates due to advanced age or significant comorbid medical problems.
Approximately 15% of patients who present with bladder cancer are found to have regional or distant metastases; approximately 30-40% of patients with invasive disease develop distant metastases despite radical cystectomy or definitive radiotherapy. Without treatment, survival is limited. Early results with single chemotherapeutic agents and, more recently, combinations of drugs have shown that a significant number of patients with metastatic bladder cancer respond partially or completely (Scher and Sternberg, 1985). The single most active agent is cisplatin, which, when used alone, produces responses in approximately 30% of patients (Yagoda, 1983). Other effective agents include methotrexate, doxorubicin, vinblastine, cyclophosphamide, and 5-fluorouracil. Response rates improve when active agents are combined. Methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) has been the most commonly used regimen for patients with advanced bladder cancer (Sternberg et al, 1988; Tannock et al, 1989). Approximately 13-35% of patients receiving such regimens attain a complete response. However, the median survival time is approximately 1 year, and the sustained survival rate is 20-25%. Treatment with MVAC is associated with substantial toxicity, including a toxic death rate of 3-4%.
Other newer agents demonstrating activity in this disease include ifosfamide, gemcitabine, paclitaxel, and gallium nitrate (Fagbemi and Stadler, 1998). A recent study demonstrated similar overall survival, time to treatment failure, and response rate for patients treated with MVAC and those treated with the newer combination of gemcitabine and cisplatin (von der Maase et al, 2000). The advantage of gemcitabine and cisplatin over MVAC is significantly lower toxicity and improved tolerability.
Once it became apparent that patients with metastatic bladder cancer could benefit from combination chemotherapy, investigators began treating patients with locally invasive (T2-T4), but not metastatic, cancer similarly. Chemotherapy can be given before planned radical cystectomy (neoadjuvant) in an attempt to decrease recurrence rates and, in selected cases, allow for bladder preservation. Approximately 22-43% of patients achieve a complete response to chemotherapy alone (Scher, 1990; Scher et al, 1988). However, additional treatment is still indicated because a substantial number of patients believed to be free of tumors after chemotherapy alone are found to have infiltrating disease at the time of surgery (Scher et al, 1989). Preliminary results from a recent randomized trial suggest that neoadjuvant chemotherapy followed by surgery may improve results when compared with surgery alone for patients with invasive disease. Alternatively, adjuvant chemotherapy may be offered to selected patients after radical cystectomy because of an increased risk of recurrence due to the presence of locally advanced disease (ie, P3, P4, or N+) (Skinner et al, 1991; Logothetis et al, 1988; Scher, 1990; Stockle et al, 1992; Stockle et al, 1995; Freiha et al, 1996). These studies suggest that patients initially managed with radical cystectomy who are found to be at an increased risk of systemic relapse due to the presence of lymph node metastases or regionally advanced disease are candidates for adjuvant chemotherapy.
Owing to high local and systemic failure rates after definitive irradiation, several investigators have explored the possibility of combining irradiation with systemic chemotherapy to decrease recurrence rates, improve patient survival, and allow bladder preservation. Trials of single-agent chemotherapy and irradiation have shown better local response rates than are found in historical series of irradiation alone (Shipley et al, 1984; Jakse, Fritsch, and Frommhold, 1985; Pearson and Raghaven, 1985).
More recently, investigators have treated patients with invasive bladder cancer with complete transurethral resection and intravenously administered combination chemotherapy followed by concomitant chemotherapy and radiation (Given et al, 1995; Chauvet et al, 1996; Shipley et al, 1997; Zietman et al, 1997; Cervek et al, 1998; Kachnic et al, 1997; Tester et al, 1996; Serretta et al, 1998). Early cystectomy is offered to those who do not tolerate chemotherapy, radiation, or both owing to toxicity and those whose cancers fail to respond to such therapy. Complete response rates to chemoradiation may be as high as 50-70% initially, with 5-year overall survival rates approaching 50-60%. However, local recurrence is common, exceeding 50% in many of these studies. Owing to invasive local recurrences, only 18-44% of patients may be alive with an intact bladder 5 years after chemoradiation. Predictors of poor outcome after combined chemoradiation for invasive bladder cancer include hydronephrosis at presentation, advanced clinical tumor stage, inability to complete the entire treatment protocol, and poor performance status. A recent study has suggested that chemoradiation may also be inappropriate for patients with bladder tumors that are p53-positive (Herr et al, 1999).
Systemic chemotherapy for locally invasive, but not metastatic, bladder cancer should not yet be considered standard therapy. The durability of the response, ultimate survival rates, and optimal candidates for the treatment regimens described will be determined only after completion of randomized studies.