Population Council presents positive results of Phase 3 trial of 1-year contraceptive vaginal ring
Today, the Population Council presented findings from a Phase 3 clinical trial that was designed to demonstrate the safety, efficacy, and acceptability of the Council’s investigational one-year contraceptive vaginal ring (CVR). The results were presented during an oral session at the 69th Annual Meeting of the American Society of Reproductive Medicine.
The study presented today (Study 300B) is part of an extensive clinical trial package that will be submitted as part of a New Drug Application to the U.S. Food and Drug Administration. The application will include two pivotal Phase 3 clinical trials conducted with more than 2,000 women across 27 study sites worldwide. Study 300B evaluated the contraceptive efficacy, safety, and acceptability of the CVR, and Study CCN006/300A evaluated efficacy and safety. The studies were conducted in partnership with the U.S. Agency for International Development (USAID); the World Health Organization (WHO); and the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Contraceptive Discovery and Development Branch.
Study 300B was a multicenter, open-label trial that involved more than 1,100 healthy, normally ovulating women across 12 study sites in Australia, Europe, Latin America, and the United States. Preliminary results suggested that the CVR - a type of long-acting, reversible contraceptive (LARC) - is as effective as other combined hormonal contraceptives in preventing pregnancy when used as directed. Preliminary results also suggested that the safety profile is consistent with that of available combined hormonal contraceptives.
In addition to evaluating contraceptive efficacy and safety, Study 300B assessed women’s acceptance of the CVR. The study found that women were highly satisfied with the ring, found it easy to use, and indicated that they would recommend it to other women. The ring was also well-accepted by their partners.
“These results add to the growing body of evidence supporting the use of the Council’s investigational one-year contraceptive vaginal ring,” said Ruth Merkatz, Ph.D., Director of Clinical Development for the Population Council’s Reproductive Health Program. “If approved by regulatory authorities, the ring will offer a unique contraceptive option: a contraceptive that is effective for one full year, is under the woman’s control, and does not require insertion by a health care professional.”
 Because the ring is effective for 13 consecutive cycles (one year) and is intended to not require refrigeration, it may be an attractive option for women in developing countries who lack convenient access to a health care facility or pharmacy, and in areas where access to reliable electricity is a challenge. Significant barriers prevent many women in developing countries from accessing a full range of contraceptive methods to meet their individual needs. These barriers may include a lack of trained providers, supply shortages, storage and electricity limitations, resistance from families or communities, and misconceptions about side effects.
Because the ring is effective for 13 consecutive cycles (one year) and is intended to not require refrigeration, it may be an attractive option for women in developing countries who lack convenient access to a health care facility or pharmacy, and in areas where access to reliable electricity is a challenge. Significant barriers prevent many women in developing countries from accessing a full range of contraceptive methods to meet their individual needs. These barriers may include a lack of trained providers, supply shortages, storage and electricity limitations, resistance from families or communities, and misconceptions about side effects.
“Our mission is to develop and help expand access to contraceptive technologies where high-quality, voluntary family planning services are scarce or nonexistent,” said Peter Donaldson, President, Population Council. “The Council’s one-year contraceptive vaginal ring is a promising new technology. We look forward to furthering its development to meet the needs of underserved women.”
###
About the Population Council’s One Year Contraceptive Vaginal Ring
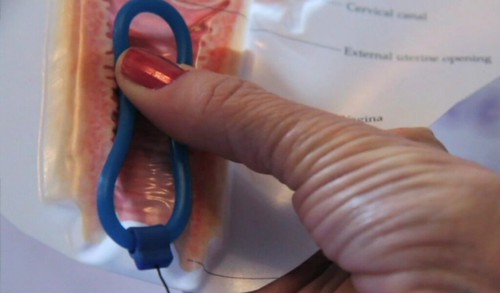 The Council developed the Nestorone® and ethinyl estradiol CVR, a one-year investigational contraceptive that delivers synthetic hormones and can provide a number of advantages compared with other methods of hormonal contraception. The ring contains ethinyl estradiol, an approved, marketed hormonal product, and Nestorone®, an investigational new chemical entity.
The Council developed the Nestorone® and ethinyl estradiol CVR, a one-year investigational contraceptive that delivers synthetic hormones and can provide a number of advantages compared with other methods of hormonal contraception. The ring contains ethinyl estradiol, an approved, marketed hormonal product, and Nestorone®, an investigational new chemical entity.
The CVR is soft, flexible, and easily inserted into the vagina by the woman herself. It will not require insertion by a health care provider. Once in place, the CVR is designed to prevent ovulation by continuously releasing a low dose of hormones through the vaginal walls and into the bloodstream. It is left in place for 21 days and removed for seven days, for up to 13 cycles (one year).
About Long-Acting Reversible Contraceptives (LARCs)
More than 200 million women in the developing world want to prevent pregnancy but are not using modern forms of contraception. Highly effective LARCs are often out of reach for women in developing countries. While several methods have been developed for short- and long-term protection against pregnancy, each woman’s needs are different and may change over the course of her lifetime.
The need for LARCs will continue to grow, and it is estimated that approximately 57 million women will be seeking LARCs by 2020. Research on policies and programs has demonstrated that the use of LARCs increases when women and health systems are provided with greater method choice. The success of these programs provides evidence for the continued expansion of such efforts.
If access to LARCs, such as intrauterine contraception (IUSs or IUDs), implants, and, in the future, longer-acting CVRs and injectables were increased, unintended pregnancies could be significantly reduced and women’s health significantly improved. Meeting contraceptive needs would help increase women’s economic empowerment and opportunities, their contributions to their families’ well-being, and economic growth.
About the Population Council
The Population Council confronts critical health and development issues - from stopping the spread of HIV to improving reproductive health and ensuring that young people lead full and productive lives. Through biomedical, social science, and public health research in 50 countries, we work with our partners to deliver solutions that lead to more effective policies, programs, and technologies that improve lives around the world. Established in 1952 and headquartered in New York, the Council is a nongovernmental, nonprofit organization governed by an international board of trustees.
###
Sasha Gruber
.(JavaScript must be enabled to view this email address)
212-339-0668
Population Council