FDA questions use of aspirin to prevent first heart attack
The U.S. Food and Drug Administration on Monday questioned the value of taking aspirin to try to ward off a first heart attack or stroke in people who have never had cardiovascular problems.
The FDA’s statement follows its decision last week to turn down a request by German drugmaker Bayer AG to change the labeling on packages in order to market aspirin’s value in preventing heart attacks in people who have never had cardiovascular disease.
Dr. Robert Temple, the agency’s deputy director for clinical science, said in an FDA “consumer update” that people should use daily aspirin therapy only after talking to a healthcare professional who can assess the benefits and risks.
Such aspirin therapy reduces the clumping action of the blood’s clotting cells, called platelets, and may prevent a heart attack, according to experts. But experts also warn that there may be serious side effects from daily use of aspirin, including internal bleeding.
“Since the 1990s, clinical data have shown that in people who have experienced a heart attack, stroke or who have a disease of the blood vessels in the heart, a daily low dose of aspirin can help prevent a re-occurrence,” Temple said in a statement on the FDA website.
But the agency added that “after carefully examining scientific data from major studies, FDA has concluded that the data do not support the use of aspirin as a preventive medication by people who have not had a heart attack, stroke or cardiovascular problems, a use that is called ‘primary prevention.’”
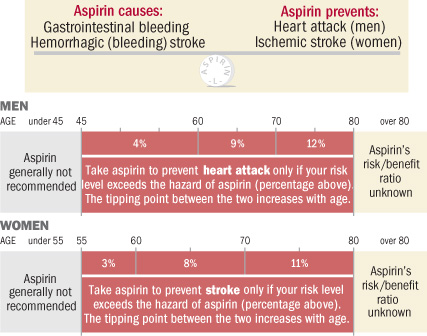 The FDA said that in these people “the benefit has not been established but risks - such as dangerous bleeding into the brain or stomach - are still present.”
The FDA said that in these people “the benefit has not been established but risks - such as dangerous bleeding into the brain or stomach - are still present.”
Some health organizations back daily aspirin therapy for people identified as having a high risk of suffering a heart attack. The American Heart Association recommends that “people at high risk of heart attack should take a daily low dose of aspirin if told to by their healthcare provider, and that heart attack survivors regularly take low-dose aspirin.”
Dr. Gregg Fonarow, a UCLA cardiologist and a representative for the American Heart Association, said the FDA has further clarified its position on whether it meets regulatory standards to allow a label for aspirin for primary prevention of heart attack and stroke.
Aspirin and heart disease
Taking aspirin helps prevent blood clots from forming in your arteries. It also lowers your risk of a stroke or heart attack.
Aspirin may be used to prevent heart or artery disease. It can also help prevent strokes.
Aspirin helps get more blood flowing to your legs. It can treat a heart attack and prevent blood clots when you have an abnormal heartbeat. You probably will take aspirin after you have treatment for clogged arteries.
You will usually take aspirin as a pill. Talk to your doctor before taking aspirin every day. Your doctor may change your dose from time to time.
Side Effects
Aspirin can have side effects: diarrhea, a skin rash, itching, nausea, or stomach pain. Before you start taking aspirin, tell your doctor if you have bleeding problems. Tell your doctor if have stomach ulcers. Also tell your doctor if you are pregnant or breastfeeding.
Fonarow added that there is the potential for confusion among the general public after the FDA statement.
“The terms that we talk about - even what is the difference between primary and secondary prevention - may be lost on the individual patient,” Fonarow said in a telephone interview.
“And I think it’s really important that before anybody initiates an aspirin regimen - and most critically before any individual considers discontinuing their aspirin regimen - that they speak specifically to their physician who knows their medical history and can help them make a better informed decision about balancing potential risks and benefits,” he said.
Preventing Heart Attack
Most heart attacks and strokes occur when the blood supply to a part of your heart muscle or brain is blocked. This usually starts with atherosclerosis, a process in which deposits of fatty substances, cholesterol, cellular waste products, calcium and other substances build up in the inner lining of an artery. This buildup is called plaque.
Plaque usually affects large and medium-sized arteries. Plaques can grow large enough to significantly reduce the blood’s flow through an artery. But most of the damage occurs when a plaque becomes fragile and ruptures. Plaques that rupture cause blood clots to form that can block blood flow or break off and travel to another part of the body. This is called an embolism.
If a blood clot blocks a blood vessel that feeds the heart, it causes a heart attack.
If a blood clot blocks a blood vessel that feeds the brain, it causes a stroke.
Aspirin “thins” the blood and helps prevent blood clots from forming. So it helps prevent heart attack and stroke.
During Heart Attack
Taking aspirin also helps during a heart attack. In fact, people having a heart attack are often given an aspirin by emergency medical services. This may take place in the ambulance or in a hospital emergency room.
Taking an aspirin as soon as symptoms start greatly improves the chance of survival.
Preventing a Second Heart Attack
By making it harder for blood clots to form, aspirin helps prevent a second heart attack. The dose of aspirin prescribed may be larger than that used to help prevent a first heart attack. Your healthcare provider will decide the right dose for you.
 Bayer said in a statement provided by company spokesman Chris Loder that “it is important that patients understand that today’s ruling does not impact the numerous cardiovascular indications for which aspirin is already approved” by the FDA.
Bayer said in a statement provided by company spokesman Chris Loder that “it is important that patients understand that today’s ruling does not impact the numerous cardiovascular indications for which aspirin is already approved” by the FDA.
“It is critical that patients who are already on aspirin therapy remain so. No one should stop or modify their aspirin regimen without first consulting with a healthcare provider. For those already on aspirin therapy, suddenly stopping can be dangerous,” the company said.
The FDA posted its statement here
###
By Will Dunham