Effects of Exercise in Diabetics
Metabolic Effects
During exercise at maximal levels energy demands may be 20-fold increased as compared to resting conditions.
In order to maintain homeostasis and prevent hypoglycemia several regulatory mechanisms are activated. Initially skeletal muscles break down their own stores of glycogen, triglycerides, and free fatty acids derived from adipose tissue. In order to mobilize extramuscular stores adjustments on a hormonal basis are necessary.
In the early phase of exercise hepatic glucose production is increased by a reduction of insulin levels in the presence of unchanged glucagon levels. In subsequent stages glucagon and catecholamine levels are elevated. As a result, glucose levels in healthy individuals remain fairly constant during exercise.
In patients with diabetes type 2 exercise of moderate or high intensity regularly decreases blood glucose levels as a result of insulin-independent activation of glucose transport (7) as well as increased insulin sensitivity (8). Due to an exaggerated counter-regulatory response in the postexercise period hyperglycemia and hyperinsulinemia is frequently encountered in patients with type 2 diabetes.
Following a meal most of the glucose contained in it is rapidly taken up by skeletal muscle and deposited as glycogen. In addition, exercise per se is a powerful stimulator of glucose uptake; part of this action is explained by the increase of skeletal muscle glucose transporter protein (GLUT4) in healthy individuals as well as in patients with type 2 diabetes; it is responsible for insulin-independent glucose transport into skeletal muscle.
Following a session of exercise with total depletion of muscular glycogen stores sufficient amounts of glucose need to be absorbed in order to replenish the stores resulting in an increase of insulin sensitivity for more than 72 hours. Conversely, after 6 months of training insulin sensitivity returned to baseline after only 72 hours of sedentary lifestyle, underlining the importance of persistent and regular exercise (9).
Regular physical activity is associated with changes in body composition with a reduction in body fat, increase in muscle mass, and maximal oxygen uptake in healthy individuals; insulin sensitivity is closely correlated to these factors. Corresponding results are obtained in patients with diabetes type 2 who engage in a structured exercise program.
Improvements of insulin sensitivity are independently correlated to a reduction in abdominal obesity and an increase in muscle cross-sectional area (10).
The benefits of exercise, however, are only maintained for short periods of time; they attenuate 3 to 6 days after the last exercise session stressing the importance of persistent lifestyle changes (11 - 13). By adding resistance training to aerobic exercise muscle mass may be increased, particularly in elderly patients who tend to loose muscle mass as a result of aging (10, 14).
Correction of Endothelial Dysfunction
Coronary macroangiopathy is preceded by endothelial dysfunction by many years; a reduction in endothelium-dependent vasodilation is a hallmark of nearly all patients with diabetes type 2 and predicts cardiovascular events (Fig. 1) (10, 15).
Endothelial-dependent responses become abnormal very early in the course of the disease and therefore they can be substituted as surrogate markers in interventional trials (16). The quality of vascular reactivity is determined by the balance between NO-production and NO inactivation.
NO is elaborated from L-arginin by the endothelial NO-synthase (eNOS) and degraded mainly by free oxygen radicals (ROS); it reaches vascular smooth muscle cell by rapid diffusion and causes a fall of intracellular Ca++ concentration resulting in vasodilation.
Endothelial dysfunction in diabetes and coronary atherosclerosis can be detected on the basis of paradox vasoconstriction following application of acetylcholine. It is the result of multifactorial process, which eventually leads to reduced concentrations of NO (17). Endothelial NO production is hampered by reduced bioavailability of the precursor L-arginin, by an increased concentration of asymmetric dimethylarginin (ADMA), which inhibits eNOS activity, as well as by alterations of the eNOS protein structure found in patients with polymorphisms (18 - 23).
Free oxygen radicals that are produced by a number of enzymes particularly in patients with diabetes are capable of destroying NO. Superoxide anions, elaborated by the
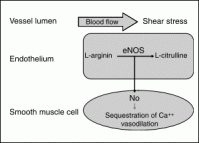 FIGURE 1 Blood flow in the coronary vessel causes deformation of the endothelial cell. In response to shear stress, endothelial NO synthase elaborates NO from L-arginin, which diffuses rapidly into smooth muscles in the vessel wall and results in sequestration of intracellular Ca++ and consecutive vasodilation.
FIGURE 1 Blood flow in the coronary vessel causes deformation of the endothelial cell. In response to shear stress, endothelial NO synthase elaborates NO from L-arginin, which diffuses rapidly into smooth muscles in the vessel wall and results in sequestration of intracellular Ca++ and consecutive vasodilation.
NADPH-oxidase and xanthin-oxidase, are responsible for NO-degradation in the first line (24, 25). In addition ROS oxidate tetrahydrobiopterin, an essential cofactor of eNOS. This leads to an uncoupling of eNOS, which now starts to produce oxygen radicals instead of NO, further aggravating endothelial dysfunction (17, 26).
To counterbalance premature NO degradation a number of enzymatic and nonenzymatic protective mechanisms are available within the endothelium; the most important are superoxide dismutase (SOD), extracellular SOD, glutathionperoxidase, catalase, and thioredoxin/thioredoxin-reductase.
A previously published study showed that endothelial dysfunction may be corrected by regular physical exercise in patients with congestive heart failure or atherosclerotic heart disease (Fig. 2) (27). Patients with stable angina pectoris were randomized between an active intervention group, which exercised on stationary bicycles, and an inactive control group.
At baseline endothelial function was assessed by intracoronary infusion of acetylcholine, which was highly abnormal in both groups. Following 4 weeks of submaximal exercise paradox vasoconstriction in response to acetylcholine was reduced by 54%. Peak blood flow velocity in response to intracoronary adenosine, representing coronary vasodilatory reserve, improved by 64%.
This finding may in part explain the reduction of anginal symptoms frequently observed in patients exercising on a regular basis.
Mobilization of Endothelial Precursor Cells
Apoptosis of endothelial cells in diabetes type 2 and atherosclerosis eventually results in loss of integrity of the endothelial lining with the consequences described above (Fig. 3).
Until recently it was accepted that repair of these defects could only be accomplished by local endothelial cells.
Recently published observations, however, indicate that certain subpopulations of bone marrow stem cells, circulatory endothelial progenitor cells (CPCs), can be mobilized in response to various stimuli, such as exercise and growth factors (28).
After leaving the bone marrow they home in to vessels with defective endothelial lining; they attach to these defective areas and become competent and functional endothelial cells.
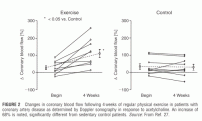 FIGURE 2 Changes in coronary blood flow following 4 weeks of regular physical exercise in patients with coronary artery disease as determined by Doppler sonography in response to acetylcholine. An increase of 68% is noted, significantly different from sedentary control patients.
FIGURE 2 Changes in coronary blood flow following 4 weeks of regular physical exercise in patients with coronary artery disease as determined by Doppler sonography in response to acetylcholine. An increase of 68% is noted, significantly different from sedentary control patients.
Their concentration in the peripheral circulation can be increased by regular physical exercise; moreover, their functional capabilities as reflected by their migratory capacity are improved, and their concentration has been associated with future cardiovascular events.
However, their survival and functional integrity in the peripheral circulation is greatly diminished by hyperglycemic states (28, 29).
Gerhard Schuler and Axel Linke
Department of Internal Medicine/Cardiology, University of Leipzig, Leipzig, Germany
REFERENCES