Health Centers > Diabetes Center > Diabetes Mellitus and Oral Health
Diabetes Mellitus and Oral Health
Introduction
Diabetes mellitus is a metabolic disease characterized by dysregulation of carbohydrate, protein, and lipid metabolism. The primary feature of this disorder is elevation in blood glucose levels (hyperglycemia), resulting from either a defect in insulin secretion from the pancreas, a change in insulin action, or both. Sustained hyperglycemia has been shown to affect almost all tissues in the body and is associated with significant complications of multiple organ systems, including the eyes, nerves, kidneys, and blood vessels.
Diabetes Mellitus and Oral Diseases
Diabetes Introduction
Diabetes Epidemiology and classification
Diabetes Pathophysiology
Clinical Presentation, Laboratory findings, and Diagnosis
Diabetes Complications
Diabetes Management
L Introduction
L Oral Agents
L Insulin
Oral Diseases and Diabetes
Periodontal Health and Diabetes
Dental Management of the Diabetic Patient
Conclusion
References
These complications are responsible for the high degree of morbidity and mortality seen in the diabetic population.
Epidemiology and classification
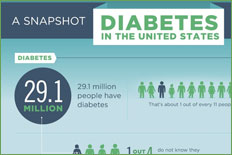
About 16 million Americans have diabetes (between 6 and 7% of the total US population). Around the world, the prevalence of diabetes is expected to double between 1994 and 2010, at which time about 240 million people will have the disease. In the United States, the incidence of diabetes rises as the population ages and as the prevalence of obesity increases.
Unfortunately, about half of those Americans with diabetes are presently unaware that they have the disease. Most dental practices have a significant number of diabetic patients in their population. Based on US prevalence data, an "average" practice would have between 60 and 70 diabetic individuals for every 1,000 patients, and 30 to 35 of these patients would be undiagnosed.
The American Diabetes Association provided the most recent classification of diabetes mellitus, in 1997. The most common forms of diabetes are termed type 1 and type 2. Type 1 diabetes was previously called insulin-dependent diabetes or juvenile diabetes while type 2 diabetes was formerly known as non-insulin-dependent diabetes or adult-onset diabetes.
The older terminology was often confusing since both insulin-dependent and non-insulin-dependent diabetic individuals may take insulin as part of their management regimen. The difference is that type 1 patients are truly dependent on insulin therapy whereas type 2 patients may benefit from insulin therapy but are not dependent on it for survival. The new classification system minimizes this confusion because it is based on the pathophysiology of the different disease types, rather than on the treatment methodology used.
Gestational diabetes occurs during pregnancy and usually resolves after delivery. Other types of diabetes may occur in individuals with certain genetic disorders, pancreatic diseases, infections, injuries to the pancreas, and endocrine diseases. Drug therapy with certain agents may also induce a diabetic state.
The Hypoglycemic States
Spontaneous hypoglycemia in adults is of two principal types: fasting and postprandial. Symptoms begin ...
Diabetes mellitus is a growing public health concern and a common chronic metabolic disease worldwide. Diabetes mellitus represents a group of metabolic diseases that are characterised by hyperglycaemia due to a total or relative lack of insulin secretion and insulin resistance or both. The metabolic abnormalities involve carbohydrate, protein and fat metabolism. Diabetes mellitus affects all age groups, but is more common in adults. The World Health Organization (WHO) has recently declared it to be a pandemic. Its prevalence has increased dramatically over the past few decades and it is expected to triple in the next decade. Diabetes mellitus is considered a leading cause of death due to its microvascular and macrovascular complications. The most common types of diabetes are type 1 (also known as insulin dependent) and type 2 (also known as non-insulin-dependent).Type 2 is the more prevalent type. Countries with the highest rates of diabetes in the Eastern Mediterranean region and the Middle East are the United Arab Emirates, Saudi Arabia, Bahrain, Kuwait and Oman. Oman is one of the countries that has a high prevalence of diabetes mellitus, especially type 2 diabetes, and its prevalence is expected to increase in the next twenty years.
Various inflammatory diseases and soft tissue pathologies in oral cavities are associated with diabetes mellitus; however, awareness of these complications is lacking worldwide. Periodontal diseases have been proposed as the sixth most prevalent complication of diabetes mellitus following the other diabetic complications. It has been reported as a more frequent oral complication of diabetes compared to other oral manifestations such as dry mouth and caries. Periodontitis is more frequent and severe in patients with diabetes with poor glycaemic control. Early identification and/or management of these oral manifestations may help in the early diagnosis of diabetes and in attaining better glycaemic control. Therefore, diabetic oral complications need to be identified and included in the ultimate care of diabetes in order to fight this chronic metabolic disease effectively.
Oral Complications and Manifestations of Diabetes Mellitus
Several soft tissue abnormalities have been reported to be associated with diabetes mellitus in the oral cavity. These complications include periodontal diseases (periodontitis and gingivitis); salivary dysfunction leading to a reduction in salivary flow and changes in saliva composition, and taste dysfunction. Oral fungal and bacterial infections have also been reported in patients with diabetes. There are also reports of oral mucosa lesions in the form of stomatitis, geographic tongue, benign migratory glossitis, fissured tongue, traumatic ulcer, lichen planus, lichenoid reaction and angular chelitis. In addition, delayed mucosal wound healing, mucosal neuro-sensory disorders, dental carries and tooth loss has been reported in patients with diabetes. The prevalence and the chance of developing oral mucosal lesions were found to be higher in patients with diabetes compared to healthy controls.
Periodontal Diseases
Periodontitis is one of the most widespread diseases in the world affecting the oral cavity, and is highly prevalent in both developed and developing countries. Periodontitis is a chronic inflammatory disorder affecting the gingivae and the periodontal tissue initiated by bacteria. The micro-flora in the dental plaque that forms daily adjacent to the teeth causes this inflammatory process. Eventually, the toxins that are released by the microorganisms in the dental plaque will start the gingival inflammation as a result of failure to remove the dental plaque on a daily basis. A periodontal pocket is formed as a result of the progression of the gingival inflammation causing the gingivae to detach from the tooth surface. This periodontal pocket is filled with bacteria and its toxins. As the disease worsens, the pocket will get deeper carrying the dental plaque until it reaches the alveolar bone that will eventually be destroyed with the periodontal attachment. This process is very common and causes destruction of periodontal tissues, loss of alveolar bone and, finally, tooth loss. There are many factors contributing to this type of inflammation beside the presence of bacteria in dental plaque; a susceptible host is one of them.
Periodontitis and Diabetes Mellitus
The link between diabetes mellitus and periodontal disease is not well recognised by the medical community. Periodontal disease has been reported with increased prevalence and severity in patients with type 1 and type 2 diabetes. The mechanism by which hyperglycaemia can induce periodontal destruction is not yet fully understood. However, there are many theories which propose factors such as advanced glycation end products, changes in collagen statue, and altered immune function that causes impaired polymorphonuclear leukocyte function which may facilitate bacterial persistence in the tissue and the accumulation of advanced glycation end products, which results from prolonged and chronic hyperglycaemia and increased secretion of pro-inflammatory cytokines such as tumour necrosis factor-α and prostaglandin E-2. The increase in collagenase activity together with the reduction in collagen synthesis will adversely influence collagen metabolism. This would result in compromised wound healing as well as periodontal tissue destruction. Recent studies indicate that periodontitis has a bidirectional effect on glycaemic control in patients with diabetes. There is a cluster of research studies, which support the hypothesis of periodontitis occurring more frequently in patients with diabetes with poor glycaemic control. In addition, there is enough evidence to support the hypothesis that poor periodontal conditions could worsen glycaemic control as well. Many studies report that diabetes is a risk factor for gingivitis and periodontitis and it is more severe with poor glycaemic control. The risk of developing periodontitis in patients with diabetes has been reported to be three times higher than the general population.
Numerous risk factors have been reported that make patients with diabetes more susceptible to periodontal disease, especially those with poor oral hygiene, poor metabolic control, longer duration of diabetes and who are smokers. Smoking was identified in many studies as being a major preventable risk factor for periodontal disease and tooth loss in the general population and in patients with diabetes. The dentist and the physician should play an important role in advising and supporting patients with diabetes regarding smoking cessation. The dentist should be engaged in counselling these patients and referring them to a specialist organisation which deals with smoking cessation.
Oral Infection
Fungal Infections
Oral candidosis is an opportunistic infection frequently caused by Candida albicans species. Many predisposing factors can lead to this infection; these include smoking, xerostomia and endocrine and metabolic diseases. Other factors were also implicated such as old age, medications, Cushing's syndrome, malignancies, and the use of dentures. Oral candidosis has been classified into primary and secondary. Primary oral candidosis is subclassified into acute (pseudomembranous and erythematous), chronic (pseudomembranous, erythematous and hyperplastic) and candida associated lesions.
Pseudomembranace candidosis is also known as oral thrush. It is characterised by the presence of a creamy white patch which, when wiped, reveals underlying erythematous and bleeding oral mucosa. The soft palate is the most commonly affected area followed by the cheek, tongue and gingivae. It could be chronic in immuno-compromised patients. Erythematous candidosis can present as acute or chronic infection. It is believed to result from the usage of steroid and broad spectrum antibiotics and mainly affects the tongue. Hyperplastic candidosis is known as candidal leukoplakia. It appears as an irregular whitish raised plaque-like lesion commonly seen in the buccal mucous membrane near the commissures.
Candida associated lesions include denture induced stomatitis, angular chelitis and median rhomboid glossitis which have mixed bacterial and fungal etiology. Denture induced stomatitis is mainly seen in full denture wearers in the underlying surface of the upper denture. Angular chelitis is seen in the lip commissures as an erythematous crusting lesion. The lesion has been reported to occur in diabetics with poor glycaemic control. Median rhomboid glossitis is seen on the dorsal surface of the tongue as adepopulated erythematous diamond-shaped patch at the midline.
The incidence of fungal infections in patients with diabetes mellitus has been recognised for many years. Candidal infection is reported to be more prevalent in patients with diabetes especially in those patients who smoke, wear dentures, have poor glycaemic control and use steroids and broad spectrum antibiotics. In addition, salivary dysfunction in patients with diabetes can also contribute to higher carriage of fungi in this group of patients. It is clear from these studies that both local and systemic predisposing factors might increase candidal carriage rate and hence increase the risk of oral candidal infection in patients with diabetes.
Bacterial Infections
Patients with diabetes are more susceptible to developing oral bacterial infections. They are well known to have an impaired defense mechanism hence considered to be immuno-compromised. Diabetics with diabetic complications and poor metabolic control are more prone to spreading and recurrent bacterial infection. Several studies have reported that patients with diabetes are more prone to deep neck bacterial infection compared to patient without diabetes. A four-year prospective study by Rao et al. investigated the severity of maxillofacial space infection of odontogenic origin, the type of micro-organism, the sensitivity of the micro-organisms to antibiotics, and the length of hospital stay of patients with diabetes compared with patients without diabetes. They concluded that the spread of the bacterial infection to the submandibular space was more common in patients and controls and that the second commonest area was the buccal space. Streptococcus species was more commonly isolated in both groups. Patients with diabetes were found to stay longer in hospital due to more severe infection and required more time to control their blood glucose levels.
Dental Caries and Tooth Loss
It is well known that patients with diabetes are susceptible to oral infections that lead to tooth decay and loss. Salivary secretion dysfunction, periodontal and sensory disorders could increase the likelihood of developing new and recurrent dental caries and tooth loss. The relationship between diabetes and development of dental caries is still unclear. It is well-known that the cleansing and buffering capacity of the saliva is diminished in patients with diabetes mellitus resulting in increased incidence of dental caries, especially in those patients who suffer from xerostomia.
Conclusion
Diabetes mellitus is a metabolic condition affecting multiple organ systems. The oral cavity frequently undergoes changes that are related to the diabetic condition, and oral infections may adversely affect metabolic control of the diabetic state. The mechanisms underlie the oral effects of diabetes share many similarities with the mechanisms that are responsible for the classic diabetic complications. The intimate relationship between oral health and systemic health in individuals with diabetes suggests a need for increased interaction between the dental and medical professionals who are charged with the management of these patients. Oral health assessment and treatment should become as common as the eye, foot, and kidney evaluations that are routinely performed as part of preventive medical therapies. Dental professionals with a thorough understanding of current medical treatment regimens and the implications of diabetes on dental care are able to help their diabetic patients achieve and maintain the best possible oral health.
References
1. National Diabetes Data Group. Diabetes in America. 2nd ed. Bethesda (MD): National Institutes of Health; 1995. NIH Publication No 95-1468.
2. Mandrup-Poulsen T. Recent advances — diabetes. BMJ 1998;316:1221-5.
3. Mealey BL. Diabetes mellitus. In: Rose LF, Genco RJ, Mealey BL, Cohen DW, editors. Periodontal medicine. Toronto, Canada: BC Decker Inc.; 2000.
4. American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183-97.
5. Newman B, Selby JV, Slemenda C, et al. Concordance for type 2 (non-insulin-dependent) diabetes mellitus in male twins. Diabetologia 1987;30:763-8.
6. Ghosh S, Schork NJ. Genetic analysis of NIDDM. Diabetes 1996;45:1-14.
7. Edelman SV. Type II diabetes mellitus. Adv Intern Med 1998;43:449-500.
8. Reaven GM. Role of insulin resistance in human disease. Diabetes 1988;37:1595-607.
9. Bogardus C, Lillioja S, Mott DM, et al. Relationship between degree of obesity and in vivo insulin action in man. Am J Physiol 1985;248:E286-E91.
10. Engelgau MM, Herman WH, Smith PJ, et al. The epidemiology of diabetes and pregnancy in the U.S., 1988. Diabetes Care 1995;18:1029-33.
11. Magee MS, Walden CE, Benedetti TJ. Influence of diagnostic criteria on the incidence of gestational diabetes and perinatal morbidity. JAMA 1993;269:609-15.
12. Langer O, Rodriguez DA, Xenakis EMJ, et al. Intensified versus conventional management of gestational diabetes. Am J Obstet Gynecol 1994;170:1036-47.
13. Charles MA, Fontboune A, Thibult N, et al. Risk factors for NIDDM in white populations: Paris Prospective Study. Diabetes 1991;40:796-9.
14. Lester E. The clinical value of glycated haemoglobin and glycated plasma proteins. Clin Biochem 1989;26:213-9.
15. Tsuji I, Nakamoto K, Hasegawa T, et al. Receiver operating characteristic analysis of fasting plasma glucose, HbA1c, and fructosamine on diabetes screening. Diabetes Care 1991;14:10757.
16. American Diabetes Association. Selfmonitoring of blood glucose (consensus statement). Diabetes Care 1993;16:605.
17. American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care 1998;21 Suppl 1:s23-31.
18. Mealey BL. Impact of advances in diabetes care on dental treatment of the diabetic patient. Compend Contin Educ Dent 1998;19:41-58.
19. Steinberg D. Diabetes and atherosclerosis. In: Porte D, Sherwin RS, editors. Diabetes mellitus. 5th ed. Stamford (CT): Appleton & Lange; 1997.
20. Steffes MW. Pathophysiology of renal complications. In: Porte D, Sherwin RS, editors. Diabetes mellitus. 5th ed. Stamford (CT): Appleton & Lange; 1997.
21. Klein R. Retinopathy and other ocular complications in diabetes. In: Porte D, Sherwin RS, editors. Diabetes mellitus. 5th ed. Stamford (CT): Appleton & Lange; 1997.
22. Brunzell JD, Chait A. Diabetic dyslipidemia: pathology and treatment. In: Porte D, Sherwin RS, editors. Diabetes mellitus. 5th ed. Stamford (CT): Appleton & Lange; 1997.
23. Brownlee M. Glycation and diabetic complications. Diabetes 1994;43:836-41.
24. Bierhaus A, Hofmann MA, Ziegler R, Nawroth PP. AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept. Cardiovasc Res 1998;37:586-600.
25. Vlassara H, Bucala R. Recent progress in advanced glycation and diabetic vascular disease: role of advanced glycation end product receptors. Diabetes 1996:45 Suppl 3:s65-6.
26. Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products. A mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res 1999;84:489-97.
27. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86.
28. Diabetes Control and Complications Trial Research Group. Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Ophthalmology 1995;102:647-61.
29. Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes management on macrovascular and microvascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol 1995;75: 894-903.
30. American Diabetes Association. Position statement. Implications of the Diabetes Control and Complications Trial. Diabetes Spectrum 1993;6:225-7.
31. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103-17.
32. U.K. Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in pateints with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53.
33. U.K. Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854-65.
34. Scheen AJ, Lefebvre PJ. Oral antidiabetic agents. A guide to selection. Drugs 1998;55:225-36.
35. Diabetes Control and Complications Trial Research Group. Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. Am J Med 1991;90:450-9.
36. Diabetes Control and Complications Trial Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997;46:271-86.
37. Sreebny LM, Yu A, Green A, Valdini A. Xerostomia in diabetes mellitus. Diabetes Care 1992;15:900-4.
38. Meurman JH, Collin HL, Niskanen L, et al. Saliva in non-insulin-dependent diabetic patients and control subjects. The role of the autonomic nervous system. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;86:69-76.
39. Fisher BM, Lamey PJ, Samaranayake LP, et al. Carriage of Candida species in the oral cavity in diabetic patients: relationship to glycaemic control. J Oral Pathol 1987;16:282-4.
40. Phelan JA, Levin SM. A prevalence study of denture stomatitis in subjects with diabetes mellitus or elevated plasma glucose levels. Oral Surg Oral Med Oral Pathol 1986;62:303-5.
41. Jones RB, McCallum RM, Kay EJ, et al. Oral health and oral health behavior in a population of diabetic clinic attenders. Community Dent Oral Epidemiol 1992;20:204-7.
42. Tenovuo J, Alanen P, Larjava H, et al. Oral health of patients with insulin dependent diabetes mellitus. Scand J Dent Res 1986;94:338-46.
43. Tavares M, DePaola P, Soparkar P, Joshipura K. Prevalence of root caries in a diabetic population. J Dent Res 1991; 70:979-83.
44. Collin HL, Uusitupa M, Niskanen L, et al. Caries in patients with non-insulin-dependent diabetes mellitus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:680-5.
45. Papapanou PN. 1996 World Workshop in Clinical Periodontics. Periodontal diseases: epidemiology. Ann Periodontol 1996;1:1-36.
46. Mealey BL. 1996 World Workshop in Clinical Periodontics. Periodontal implications: medically compromised patients. Ann Periodontol 1996;1:256-321.
47. Gusberti FA, Syed SA, Bacon G, et al. Puberty gingivitis in insulin-dependent diabetic children. J Periodontol 1983; 54:714-20.
48. Ervasti T, Knuuttila M, Pohjamo L, Haukipuro K. Relation between control of diabetes and gingival bleeding. J Periodontol 1985;56:154-7.
49. Karjalainen KM, Knuuttila MLE. The onset of diabetes and poor metabolic control increases gingival bleeding in children and adolescents with insulin-dependent diabetes mellitus. J Clin Periodontol 1996;23:1060-7.
50. Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol 1991;62:123-30.
51. Shlossman M, Knowler WC, Pettitt DJ, Genco RJ. Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc 1990;121:532-6.
52. Taylor GW, Burt BA, Becker MP, et al. Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol 1998;69:76-83.
53. Seppala B, Seppala M, Ainamo J. A longitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J Clin Periodontol 1993;20:161-5.
54. Tervonen T, Oliver RC. Long-term control of diabetes mellitus and periodontitis. J Clin Periodontol 1993;20:431-5.
55. Zambon JJ, Reynolds H, Fisher JG, et al. Microbiological and immunological studies of adult periodontitis in patients with non-insulin dependent diabetes mellitus. J Periodontol 1988;59:23-31.
56. Sastrowijoto SH, Hillemans P, van Steenbergen TJ, et al. Periodontal condition and microbiology of healthy and diseased periodontal pockets in type 1 diabetes mellitus patients. J Clin Periodontol 1989;16:316-22.
57. Ficara AJ, Levin MP, Grower MF, Kramer GD. A comparison of the glucose and protein content of gingival crevicular fluid from diabetics and nondiabetics. J Periodontal Res 1975;10:171-5.
58. Nishimura F, Takahashi K, Kurihara M, et al. Periodontal disease as a complication of diabetes mellitus. Ann Periodontol 1998;3:20-9.
59. Frantzis TG, Reeve CM, Brown AL. The ultrastructure of capillary basement membranes in the attached gingiva of diabetic and non-diabetic patients with periodontal disease. J Periodontol 1971;42:406-11.
60. Seppala B, Sorsa T, Ainamo J. Morphometric analysis of cellular and vascular changes in gingival connective tissue in long-term insulin-dependent diabetes. J Periodontol 1997;68:1237-45.
61. Schmidt AM, Weidman E, Lalla E, et al. Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: a potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res 1996;31:508-15.
62. Iacopino AM. Diabetic periodontitis: possible lipid-induced defect in tissue repair through alteration of macrophage phenotype and function. Oral Dis 1995;1:214-29.
63. Manoucher-Pour M, Spagnuolo PJ, Rodman HM, Bissada NF. Comparison of neutrophil chemotactic response in diabetic patients with mild and severe periodontal disease. J Periodontol 1981;52:410-5.
64. McMullen JA, Van Dyke TE, Horoszewicz HU, Genco RJ. Neutrophil chemotaxis in individuals with advanced periodontal disease and a genetic predisposition to diabetes mellitus. J Periodontol 1981;52:167-73.
65. Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol 1996;1:821-78.
66. Salvi GE, Collins JG, Yalda B, et al. Monocytic TNF-α secretion patterns in IDDM patients with periodontal diseases. J Clin Periodontol 1997;24:8-16.
67. Salvi GE, Yalda B, Collins JG, et al. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontol 1997;68:127-35.
68. Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal disease. J Periodontol 1993;64:474-84.
69. Golub LM, Lee H-M, Ryan ME. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res 1998;12:12-26.
70. Taylor GW, Burt BA, Becker MP, et al. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol 1996;67:1085-93.
71. Thorstensson H, Kuylensteirna J, Hugoson A. Medical status and complications in relation to periodontal disease experience in insulin-dependent diabetics. J Clin Periodontol 1996;23:194-202.
72. Miller LS, Manwell MA, Newbold D, et al. The relationship between reduction in periodontal inflammation and diabetes control: a report of 9 cases. J Periodontol 1992;63:843-8.
73. Grossi SG, Skrepcinski FB, DeCaro T, et al. Response to periodontal therapy in diabetics and smokers. J Periodontol 1996;67:1094-12.
74. Grossi SG, Skrepcinski FB, DeCaro T, et al. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol 1997;68:713-9.
75. Aldridge JP, Lester V, Watts TLP, et al. Single-blind studies of the effects of improved periodontal health on metabolic control in type 1 diabetes mellitus. J Clin Periodontol 1995; 22:271-5.
76. Christgau M, Palitzsch KD, Schmalz G, et al. Healing response to non-surgical periodontal therapy in patients with diabetes mellitus: clinical, microbiological, and immunological results. J Clin Periodontol 1998;25:112-24.
77. Westfelt E, Rylander H, Blohme G, et al. The effect of periodontal therapy in diabetics. Results after 5 years. J Clin Periodontol 1996;23:92-100.
78. Tervonen T, Karjalainen K. Periodontal disease related to diabetic status. A pilot study of the response to periodontal therapy in type 1 diabetes. J Clin Periodontol 1997;24:505-10.
79. Rees TD. The diabetic dental patient. Dent Clin North Am 1994;38:447-63.
80. American Academy of Periodontology. Diabetes and periodontal diseases. Position paper. J Periodontol 1999;70:935-49.