New signaling pathway discovered in HER2-positive breast cancer, and 2 powerful drug targets
One of the most promising ideas in cancer treatment is to apply a lesson learned in the fight against AIDS (Acquired Immune Deficiency Syndrome): simultaneously attacking a pathological process at different points of weakness can, in some cases, deal a knock-out blow. Just as the so-called AIDS “cocktail” directs multiple agents against multiple targets, so too might future anti-cancer cocktails be directed at multiple, highly specific targets in known cancer pathways. One key in cancer is knowing precisely which targets to hit, in which combinations, for the illness takes many different forms and works through a stunning variety of biological mechanisms.
A team at Cold Spring Harbor Laboratory (CSHL) has published in the Journal of Biological Chemistry results of experiments that lay bare a previously unknown pathway activated in a highly lethal form of breast cancer. The pathway, they discovered, contains at least two potentially powerful drug targets, according to the team leader, CSHL Professor Nicholas K. Tonks.
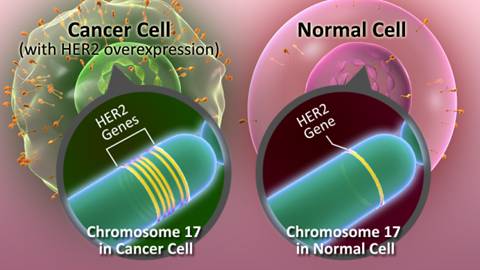
The breast cancer type is called HER2-positive. Affecting about one breast cancer patient in four, it is characterized by tumor cells overexpressing a signaling protein called HER2. The drug Herceptin, which targets HER2, is an effective first-line treatment for about one-third of women with HER2-positive breast cancer, but in most cases, resistance to the treatment develops within a year.
HER2-positive breast cancer, which is associated with poor prognosis, has been traced to an excess of signaling through receptors, or docking ports, called HER2 found on the surface of certain mammary epithelial cells. (Scientists also use the name ERBB2 to describe this surface receptor). When an activating protein docks at the receptor, a cascade of signals is sent inside breast cells. Ultimately, these signals change the expression of genes in the cell nucleus, causing the cell to grow abnormally. Herceptin, when it works, blocks the ability of HER2 receptors to send these aberrant, growth-inducing signals inside the cell.
Genes contain the recipes for the various proteins a cell needs to stay healthy and function normally. Some genes and the proteins they make can influence how a breast cancer behaves and how it might respond to a specific treatment. Cancer cells from a tissue sample can be tested to see which genes are normal and abnormal. The proteins they make can also be tested.
HER2 (human epidermal growth factor receptor 2) is one such gene that can play a role in the development of breast cancer. Your pathology report should include information about HER2 status, which tells you whether or not HER2 is playing a role in the cancer.
The HER2 gene makes HER2 proteins. HER2 proteins are receptors on breast cells. Normally, HER2 receptors help control how a healthy breast cell grows, divides, and repairs itself. But in about 25% of breast cancers, the HER2 gene doesn’t work correctly and makes too many copies of itself (known as HER2 gene amplification). All these extra HER2 genes tell breast cells to make too many HER2 receptors (HER2 protein overexpression). This makes breast cells grow and divide in an uncontrolled way.
Breast cancers with HER2 gene amplification or HER2 protein overexpression are called HER2-positive in the pathology report. HER2-positive breast cancers tend to grow faster and are more likely to spread and come back compared to HER2-negative breast cancers. But there are medicines specifically for HER2-positive breast cancers.
 But often Herceptin does not work at all, and even when effective, it stops working after a while. With this in mind, Tonks and a team that included Mathangi Ramesh, a Ph.D. student in his lab and first author of the new paper, set out to find weaknesses “downstream” of the HER2 receptor, inside breast cancer cells. In particular, they looked at the possible involvement in the HER2 pathway of a class of enzymes called protein tyrosine phosphatases, or PTPs. Transmission of signals in a cell is controlled by the coordinated activity of two families of enzymes: protein tyrosine kinases, which add a phosphate group to proteins, and protein tyrosine phosphatases, which remove them. Dr. Tonks purified the first-discovered member of the PTP family of enzymes, called PTP1B, in1988. It has been shown that PTP1B has a role in regulating proliferation in HER2-positive breast cancer cells.
But often Herceptin does not work at all, and even when effective, it stops working after a while. With this in mind, Tonks and a team that included Mathangi Ramesh, a Ph.D. student in his lab and first author of the new paper, set out to find weaknesses “downstream” of the HER2 receptor, inside breast cancer cells. In particular, they looked at the possible involvement in the HER2 pathway of a class of enzymes called protein tyrosine phosphatases, or PTPs. Transmission of signals in a cell is controlled by the coordinated activity of two families of enzymes: protein tyrosine kinases, which add a phosphate group to proteins, and protein tyrosine phosphatases, which remove them. Dr. Tonks purified the first-discovered member of the PTP family of enzymes, called PTP1B, in1988. It has been shown that PTP1B has a role in regulating proliferation in HER2-positive breast cancer cells.
In the new work, the team looked at 37 members of the PTP family to see if any of them also helped to regulate some part of the HER2 pathway. Using a three-dimensional model of mammary cell development created by Dr. Sentil Muthuswamy, formerly of CSHL and now at the University of Toronto, the team tested to see if abnormal growth initiated by HER2 signaling would be either enhanced or reduced if any of the 37 PTPs was experimentally “knocked down.” They went through the list methodically, one at a time. This led them to focus on one in particular, called PTPD2.
Tonks and colleagues found that when PTPD2 was absent in mammary cells grown in 3-D culture, the cells failed to grow abnormally, even when the HER2 signaling pathway was activated. PTPD2, therefore, is what scientists call a “positive regulator” of the pathway. Without it, abnormal growth of the type seen in HER2-positive cancers does not occur. Conversely, a future drug that prevents PTPD2 from acting in cancer cells could generate therapeutic results - by preventing or reducing HER2 signaling.
Genes and Breast Cancer
Breast cancer is often discussed as a general condition, but there are several different types that require different treatments.
One way to distinguish breast cancer cells is through your genes. When you’re diagnosed with breast cancer, your doctor will test the cancerous cells to determine their genetic makeup.
Read through to learn what it means if the genes in your cancer cells have more HER2 protein than they should.
HER2 Basics
HER2 is a protein that stimulates the growth of breast cancer cells. It can be found in your blood and urine. Sometimes it’s referred to as a “tumor marker.”
Tumor markers like HER2 can’t be used for cancer diagnosis, but they can provide other important information. For example, the presence of HER2 can help your doctor predict how your breast cancer is likely to respond to treatment.
How Many Are HER2-Positive?
The Mayo Clinic estimates that about 20 percent of breast cancers are HER2-positive. Younger women are more likely to be HER2-positive than older women.
HER2-positive breast cancer tends to be more aggressive and to spread more quickly than other cancers. That’s why it’s important to find out if the cancer cells in your body contain this protein.
Testing for HER2
Your doctor will order a lab test to determine if your cancer is HER2-positive. The American Cancer Society (ACS) advises that all patients diagnosed with breast cancer should get tested for HER2.
If your breast cancer is HER2-positive, you have a much better chance of successful treatment with methods that target the HER2 protein specifically.
In other experiments designed to determine how PTPD2 operates in HER2-activated breast cancer cells, the team found an “interaction partner,” a lipid called phosphatidic acid, or PA. PTPD2 binds to PA, the team discovered, and becomes more active as a consequence. Another series of experiments demonstrated that when the enzyme that generates PA in the cell, called PLD2, is targeted with an existing small-molecule drug, abnormal growth does not occur in mammary cells in which the HER2 pathway is activated. Thus PLD2 is also a potential drug target.
Perhaps most exciting, the CSHL team was able to show a specific series of relationships with direct implications for possible future combination drug development. “In this work, Mathangi Ramesh has found a new pathway - a signaling pathway downstream of HER2 that we didn’t know about before,” Tonks says. “Two components of the pathway, the phosphatase PTPD2 and the lipid PA, are together required for HER2 signaling to function in mammary epithelial cells.” Specifically, they are required in processes in which mammary cells lose their normal polarity, or spatial orientation; and in which the cells lose the ability to commit preprogrammed suicide, or apoptosis, upon detection of gross abnormalities. Both of these flaws occur in HER2-positive breast cancer cells.
Both PTPD2 and PLD2 might therefore be the targets of future drugs. So too might the phosphatase PTP1B, which in prior work was shown to be required for a third aspect of HER2-related carcinogenesis - proliferation - that neither PTPD2 or PLD2 specifically affects, as shown in the current phase of the research.
“If you can use combination approaches, hitting multiple targets within the cell to reduce the activity of each, and you see a synergistic effect between them, you may be able to overcome some of their harmful effects in HER2-positive cancer, and perhaps also resistance,” says Tonks. “That is our goal.”
###
The research described here was supported by the National Institutes of Health; The Gladowksy Breast Cancer Foundation; The Don Monti Memorial Research Foundation; Hansen Memorial Foundation; West Islip Breast Cancer Coalition for Long Island; Glen Cove CARES; Find a Cure today (FACT); Constance Silveri; Robertson Research Fund; Masthead Cover Yacht Club Carol Marcincuk Fund.
“A novel phosphatidic acid-protein tyrosine phosphatase D2 axis is essential for ERBB2 signaling in mammary epithelial cell” appeared online April 10, 2015 in Journal of Biological Chemistry. The authors are: Mathangi Ramesh, Navasona Krishnan, Senthil K. Muthuswamy and Nicholas K. Tonks.
About Cold Spring Harbor Laboratory
Celebrating its 125th anniversary in 2015, Cold Spring Harbor Laboratory has shaped contemporary biomedical research and education with programs in cancer, neuroscience, plant biology and quantitative biology. Home to eight Nobel Prize winners, the private, not-for-profit Laboratory is more than 600 researchers and technicians strong. The Meetings & Courses Program hosts more than 12,000 scientists from around the world each year on its campuses in Long Island and in Suzhou, China. The Laboratory’s education arm also includes an academic publishing house, a graduate school and programs for middle and high school students and teachers.
###