Health Centers > Diabetes Center > Treatment of Diabetes - Geriatric Medicine
Treatment of Diabetes - Geriatric Medicine
Sites of Care
Care of the older diabetic patient, similar to the care of other complex geriatric patients, has become a multidisciplinary issue with very high stakes in terms of vascular, renal, and ocular disability. Recent studies have demonstrated the value of careful management on the improvement in patient outcomes, and large-scale studies are under way to deter or prevent the emergence of clinical disease.
Treatment of Diabetes - Geriatric Medicine
Sites of Care
Demographics, Epidemiology, and Risk Factors
Specific Clinical Patterns with Aging
Pathogenesis of Age-Associated Glucose Intolerance
Pathogenesis of Diabetes Mellitus in the Elderly
Clinical Presentation
Diagnosis and Differential Diagnosis of Diabetes Mellitus in the Elderly
Prognosis and Course of Illness
Glucose Control
United Kingdom Prospective Diabetes Study
Blood Pressure Control and Complications in Type 2 Diabetes
Treatment and Management of Diabetes Mellitus
L Oral Hypoglycemic Agents
L Diet
L Exercise
L Agents Increasing Insulin Secretion
L Agents Increasing Insulin Action
L Agents Slowing Carbohydrate Processing in the Gut
L Combination Therapy
Management During Terminal Phases of the Illness: The Elderly Nursing Home Patient
Management of the Hospitalized Diabetic Patient
Prevention of Clinical Diabetes Mellitus
References
Increasingly, the focus of care is planning a comprehensive, multidisciplinary treatment and assessment program designed to prevent end-organ injury and to intervene early in the course of illness. It is an encouraging time to be involved in the treatment of diabetic patients, particularly outpatients treated early in the course of their illness. Improved care of the vascular and renal complications in those with advanced disease has also produced promise for their higher quality of life.
Demographics, Epidemiology, and Risk Factors
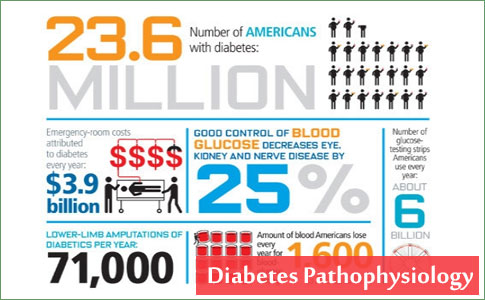
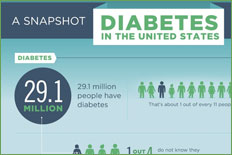
Diabetes mellitus prevalence increases with age, and the numbers of older persons with diabetes are expected to grow as the elderly population increases in number (Figure 46.1). The National Health and Nutrition Examination Survey (NHANES III) demonstrated that, in the population over 65 years old, almost 18% to 20% have diabetes. Of great diagnostic and clinical significance is that one-half of those with diabetes mellitus are not aware they have the disease. Other abnormalities in carbohydrate metabolism that have been observed include an additional 20% to 25% older patients meeting the criteria for impaired glucose tolerance. These unknown diabetic individuals and those potentially at risk were uncovered using glucose tolerance tests, which are very sensitive to abnormalities in carbohydrate economy.
Prevalence of Gestational Diabetes Mellitus
Recent data show that gestational diabetes mellitus (GDM) prevalence has increased by ~10-100% in several race/ethnicity groups during the past 20 years. A true increase in the prevalence of GDM, aside from its adverse consequences for infants in the newborn period, might also reflect or contribute to the current patterns of increasing diabetes and obesity, especially in the offspring. Therefore, the public health aspects of increasing GDM need more attention.
The frequency of GDM usually reflects the frequency of type 2 diabetes in the underlying population. Established risk factors for GDM are advanced maternal age, obesity, and family history of diabetes.
The Hypoglycemic States
Spontaneous hypoglycemia in adults is of two principal types: fasting and postprandial. Symptoms begin ...
Unquestionably, there are ethnic differences in the prevalence of GDM. In the U.S., Native Americans, Asians, Hispanics, and African-American women are at higher risk for GDM than non-Hispanic white women. In Australia, GDM prevalence was found to be higher in women whose country of birth was China or India than in women whose country of birth was in Europe or Northern Africa. GDM prevalence was also higher in Aboriginal women than in non-Aboriginal women. In Europe, GDM has been found to be more common among Asian women than among European women. The proportion of pregnancies complicated by GDM in Asian countries has been reported to be lower than the proportion observed in Asian women living in other continents. In India, GDM has been found to be more common in women living in urban areas than in women living in rural areas.
The trend toward older maternal age, the epidemic of obesity and diabetes, and the decrease in physical activity and the adoption of modern lifestyles in developing countries may all contribute to an increase in the prevalence of GDM. Because GDM is associated with several perinatal complications, and because women with GDM and their offspring are also at increased risk of developing diabetes later in life, it is critical to assess trends in GDM prevalence to allocate appropriate resources to perinatal management and postpartum diabetes prevention strategies. Characterizing trends in GDM might also help to understand possible mechanisms for the increase of obesity and type 2 diabetes, especially in children. Recent data show that GDM prevalence has increased by ~ 16 - 127% in several race/ethnicity groups during the past 20 years. These variations may depend on differences in methodology and study populations across studies. Methodological issues are described below as well as studies of trends in GDM. Some studies calculated the "cumulative incidence" (defined as the percentage of pregnancies in which GDM was recognized) because GDM frequency was calculated among screened pregnancies regardless of whether they delivered an infant. However, most of the studies identified only women who delivered, and therefore they calculated the "prevalence" of GDM at delivery. For simplicity, the term "prevalence" of GDM will be used for all studies, since the GDM cumulative incidence estimates are similar to the prevalence estimates, given the small number of preggnancies that were screened but did not deliver an infant.
The incidence of diabetes mellitus is approximately 2 per 1000 among those older than 45 and increases for those individuals more than 75 years old. Prevalence is much higher in older Hispanics, African Americans, Native Americans (Indians), Scandinavians, Japanese, and Micronesians.
Diabetes mellitus is the most common chronic endocrine disorder, affecting an estimated 5% to 10% of the adult population in industrialized Western countries, Asia, Africa, Central America and South America, and it has a large impact on society. The International Diabetes Federation (IDF) estimated that there were 151 million people with diabetes in 2000. Despite methodological differences, this was similar to the present estimate for a comparable population of 147 million. The IDF has subsequently released estimates of the numbers of people with diabetes for 2003 of 194 million and forecasts for 2025 of 334 million. The clinical characteristics of the diabetic population and their comorbidities have been obtained mainly from hospitals or community-based surveys. The accompanying shift in lifestyle to more sedentary activity with higher-fat diets and resultant obesity apparently underlies much of the increased prevalence of diabetes mellitus. The Saudi population is over 18 million and is rapidly growing. Previous national health surveys have provided information on the prevalence in the northwestern, southwestern, northern, eastern and central provinces.
Individuals with diabetes mellitus who are older than 65 usually have noninsulin-dependent diabetes (NIDDM). Insulin-dependent diabetes mellitus (IDDM) accounts for only 5% to 10% newly diagnosed diabetes mellitus in late life. In addition, a small proportion of older individuals who initially have NIDDM appear to become insulin dependent over time. A few clues as to who will require insulin exist. Ketosis at the time of diagnosis suggests that insulin therapy will be necessary. However, some elderly individuals with diabetes and ketosis can subsequently be treated with oral agents. The human leukocyte antigen (HLA)-DR3 serotype is more common in older adults who require insulin treatment. The frequency of antibodies to islet cells in older diabetic patients is not increased.
1. Siegel JS. Recent and prospective trends for the elderly population and some implications for health care. In: Haynes S, Feinleib M, eds. Second Conference on the Epidemiology of Aging. DHHS (NIH) 80-969. Washington, DC: U.S. Government Printing Office; 1980.
2. Guralnik JM, Fitzsimmons SC. Aging in America: a demographic perspective. Cardiol Clin. 1986;4:175-183.
3. Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults. Diabetes Care. 1998;21:518-524.
4. Harris MI. Undiagnosed NIDDM: clinical and public health issues. Diabetes Care. 1993;16:642-652.
5. Kilvert A, Fitzgerald MG, Wright AD, et al. Clinical characteristics and aetiological classification of insulin-dependent diabetes in the elderly. Q J Med. 1986;60:865- 872.
6. Wachtel TJ, Tetu-Mouradjian LM, Goldman DL, Ellis SE, O'Sullivan PS. Hyperosmolarity and acidosis in diabetes mellitus: a three-year experience in Rhode Island. J Gen Intern Med. 1991;6:495-502.
7. Abbott RD, Donahue RP, MacMahon SW, Reed DM, Yano K. Diabetes and the risk of stroke. The Honolulu Heart Program. JAMA. 1987;257:949-952.
8. Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312-318.
9. Shlossman M, Knowler WC, Pettitt DJ, Genco RJ. Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc. 1990;121:532-536.
10. Centers for Disease Control and Prevention. Diabetes Surveillance, 1993. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 1993.
11. Elahi D, Muller DC. Carbohydrate metabolism in the elderly. Eur J Clin Nutr. 2000;54:S112-S120.
12. Davidson MB. The effect of aging on carbohydrate metabolism: a review of the English literature and a practical approach to the diagnosis of diabetes mellitus in the elderly. Metabolism. 1979;28:688-705.
13. Jackson RA, Blix PM, Matthews JA, et al. Influence of aging on glucose homeostasis. J Clin Endocrinol Metab. 1982;55:840-848.
14. Minaker KL. What diabetologists should know about elderly patients. Diabetes Care. 1990;13(suppl 2):34-46.
15. Jackson RA, Hawa MI, Roshania RD, et al. Influence of aging on hepatic and peripheral glucose metabolism in humans. Diabetes. 1988;37:119-129.
16. Chen M, Bergman RN, Pacini G, et al. Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin Endocrinol Metab. 1985;60:13-20.
17. DeFronzo RA. Glucose intolerance and aging. Diabetes Care. 1981;4:493-501.
18. Lipson LG. Diabetes in the elderly: diagnosis, pathogenesis, and therapy. Am J Med. 1986;80(suppl 5A):10-21.
19. Fink RI, Kolterman OG, Kao M, et al. The role of the glucose transport system in the postreceptor defect in insulin action associated with human aging. J Clin Endocrinol Metab. 1984;58:721-725.
20. Belfiore F, Vagnoni G, Napoli E, Rabuazzo M. Effect of aging on key enzymes of glucose metabolism in human adipose tissue. J Mol Med. 1977;2:89-95.
21. Trischitta V, Reaven GM. Evidence of a defect in insulin-receptor recycling in adipocytes from older rats. Am J Physiol. 1988;254:E38-E44.
22. Forbes GB, Reina JC. Adult lean body mass declines with age: some longitudinal observations. Metabolism. 1977;19:653-663.
23. Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91-106.
24. Seals DR, Hagberg JM, Allen WK, et al. Glucose tolerance in young and older athletes and sedentary men. J Appl Physiol.l984;56:1521-1525.
25. Chen M, Bergman RN, Porte D Jr. Insulin resistance and beta-cell dysfunction in aging: the importance of dietary carbohydrate. J Clin Endocrinol Metab. 1988;67:951-957.
26. Donahue RP, Abbott RD, Reed DM, et al. Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry: Honolulu Heart Program. Diabetes. 1987;36:689-692.
27. Agner E, Thorsteinsson B, Eriksen M. Impaired glucose tolerance and diabetes mellitus in elderly subjects. Diabetes Care. 1982;5:600-604.
28. Skarfors ET, Selinus KI, Lithell HO. Risk factors for developing noninsulin-dependent diabetes. A 10-year follow-up of men in Uppsala. Br Med J. 1991;303:755-760.
29. Vaag A, Henriksen JE, Madsbad S, Holm N, Beck-Nielsen H. Insulin secretion, insulin action, and hepatic glucose production in identical twins discordant for non-insulin-dependent diabetes mellitus. J Clin Investig. 1995;95:690- 698.
30. Tibblin G, Adlerberth A, Lindstedt GB, Bjorntorp P. The pituitary-gonadal axis and health in elderly men. Diabetes. 1996;45:1605-1609.
31. Goodman-Gruen D, Barrett-Connor E. Sex hormone-binding globulin and glucose tolerance in post-menopausal women. The Rancho Bernardo Study. Diabetes Care. 1997;20:645-649.
32. Feskens EJM, Bowles CH, Kromhout D. Carbohydrate intake and body mass index relation to the risk of glucose in tolerance in an elderly population. Am J Clin Nutr. 1991;54:136-140.
33. Feskens EJM, Virtanen SM, Rasanen L, et al. Dietary factors determining diabetes and impaired glucose tolerance. Diabetes Care. 1995;18:1104-1111.
34. Marshall JA, Weiss NS, Hamman RF. The role of dietary fiber in the etiology of non-insulin-dependent diabetes mellitus. The San Luis Valley diabetes study. Ann Epidemiol. 1993;3:18-26.
35. Salmeron J, Asherio A, Rimm EB, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545-550.
36. Lipton RB, Liao Y, Cao G, Cooper RS, McGee D. Determinants of incident non-insulin-dependent diabetes mellitus among blacks and whites in a national sample. The NHANES I Epidemiologic follow-up study. Am J Epidemol. 1993;138:826-964.
37. Cassano PA, Rosner B, Vokonas PS. Obesity and body fat distribution in relation to the incidence of non-insulin-dependent diabetes mellitus. Am J Epidemiol. 1992;136:1474-1486.
38. Travia D, Bonora E, Cacciatori V, et al. Study of some putative pathogenic factors of diabetes mellitus in the elderly. Arch Gerontol Geriatr. 1991;2(supp1 2):219-222.
39. Morris RD, Rimm AA. Association of waist to hip ratio and family history with the prevalence of NIDDM among 25,272 adult, white females. Am J Public Health. 1991;81:507-509.
40. Manson JE, Nathan DM, Krolewski AS, Stampter MJ, Willett HWC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. JAMA. 1992;268:63-67.
41. Mykkanen L, Kuusisto J, Pyorala K, Laakso M. Cardiovascular disease risk factors as predictors of type 2 (non-insulin-dependent) diabetes mellitus in elderly subjects. Diabetologia. 1993;36:553-559.
42. Helmrich SP, Ragland DR, Leung RW, Paffenburger RS. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med. 1991;325:147- 195.
43. Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM. Diabetes. 1997;46:701-710.
44. Gurwitz J, Field TS, Glynn RJ, et al. Risk factors for NIDDM requiring treatment in the elderly. J Am Geriatr Soc. 1994;42:1235-1240.
45. Mooy JM, Grootenhuis PA, de Vries H, et al. Prevalence and determinants of glucose intolerance in a Dutch Caucasian population. Diabetes Care. 1995;18:1270-1273.
46. Stolk RP, Pols HA, Lamberts SWJ, de Jong PTVM, Hofman A, Grobbee DE. Diabetes mellitus, impaired glucose tolerance, and hyperinsulinemia in an elderly population. Am J Epidemiol. 1997;145:24-32.
47. Meneilly GS, Hards L, Tessier D, Elliott T, Tildesley H. NIDDM in the elderly. Diabetes Care. 1996;19:1320-1325.
48. Arner P, Pollare T, Lithell H. Different aetiologies of type 2 (non-insulin-dependent) diabetes mellitus in obese and non-obese subjects. Diabetologia. 1991;4:483-487.
49. Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care. 1996;19:1019-1030.
50. Forbes A, Elliott T, Tildesley H, Finegood D, Meneilly GS. Alterations in non-insulin-mediated glucose uptake in the elderly patient with diabetes. Diabetes. 1998;47:1915-1919.
51. Meneilly GS, Tessier D. Diabetes in the elderly. In: Morley JE, van den Berg L, eds. Contemporary Endocrinology: Endocrinology of Aging. Totowa, NJ: Humana Press; 1997: 181-203.
52. McCarthy MI, Hitman GA, Hitchins M, et al. Glucokinase gene polymorphisms: a genetic marker for glucose intolerance in cohort of elderly Finnish men. Diabet Med. 1993;10:198-204.
53. Laakso M, Malkki M, Kekalainen P, Kuusisto J, Mykannen L, Deeb S. Glucokinase gene variants in subjects with late-onset NIDDM and impaired glucose tolerance. Diabetes Care. 1995;18:398-400.
54. Obermajer-Kusser B, White MF, Pongratz DE, et al. A defective intramolecular autoactivation cascade may cause the reduced kinase activity of the skeletal muscle insulin receptor from patients with non-insulin-dependent diabetes mellitus. J Biol Chem. 1989;264:9497-9504.
55. Morley JE, Kaiser FE. Unique aspects of diabetes mellitus in the elderly. Clin Geriatr Med. 1990;6:693-702.
56. Tattersall RB. Diabetes in the elderly—a neglected area. Diabetologia. 1984;27:167-173.
57. James WD, Odom RB, Goette DKL. Bullous eruption of diabetes mellitus. Arch Dermatol. 1980;116:1191-1192.
58. Friedman NA, LeBan NB. Periarthrosis of the shoulder associated with diabetes mellitus. Am J Phys Med Rehabil. 1989;68:12-14.
59. Neil MAW, Dawson JA, Baker JE. Risk of hypothermia in elderly patients with diabetes. Br Med J. 1986;293:416-418.
60. Ellenberg M. Diabetic neuropathic cachexia. Diabetes. 1974;23:418-423.
61. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183-1197.
62. World Health Organization. Diabetes Mellitus: Report of a WHO Study Group. Geneva; WHO; 1985.
63. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039-1057.
64. Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15:539-553.
65. Harris M, Flegal K, Cowie C, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults. The third national health and nutrition examination survey, 1988-1994. Diabetes Care. 1998;21:518-524.
66. Bennett PH, Rushfroth NB, Miller M, Lecompte PM. Epidemiologic studies of diabetes in the Pima Indians. Recent Prog Horm Res. 1976;32:333-376.
67. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
68. UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
69. UKPDS Group. Effect of intensive blood-glucose control with metformin on complications on overweight patients with type 2 diabetes (UKDPS 34). Lancet. 1998;352:854- 865.
70. UKPDS Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J. 1998;317:703-713.
71. Rowe JW, Andres R, Tobin JD, et al. The effect of age on creatinine clearance in man: a cross-sectional and longitudinal study. J Gerontol. 1976;31:513-563.
72. Mackenzie RA, Phillips LH II. Changes in peripheral and central nerve conduction with aging. Clin Exp Neurol. 1981;18:109-116.
73. Naliboff BD, Rosenthal M. Effects of age on complications in adult onset diabetes. J Am Geriatr Soc. 1989;27:838- 842.
74. Albert MS. Cognitive function. In: Albert MS, Moss MB, eds. Geriatric Neuropsychology. New York: Guilford; 1988: 33-53.
75. Ciocon JO, Potter JF. Age-related changes in human memory: normal and abnormal. Geriatrics. 1988;43(10):43-48.
76. Einstein GO, McDaniel MA. Normal aging and prospective memory. J Exp Psychol Learn Mem Cogn. 1990;16:717-726.
77. Morrow LA, Halter JB. Treatment of diabetes mellitus in the elderly. In: Weir GC, ed. Joslin's Diabetes Mellitus,13th Ed. Philadelphia; Lea & Febiger; 1993.
78. American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 1989;12:365-368.
79. Wetzler HP, Synder JW. Linking pharmacy and laboratory data to assess the appropriateness of care in patients with diabetes. Diabetes Care. 2000;23:1637-1641.
80. Reaven GM. Beneficial effect of moderate weight loss in older patients with non-insulin-dependent diabetes mellitus poorly controlled with insulin. J Am Geriatr Soc. 1985;33:93-95.
81. Holloszy JO, Schultz J, Kursnierkiewicz J, et al. Effects of exercise on glucose tolerance and insulin resistance. Brief review and some preliminary results. Acta Med Scand Suppl. 1986;711:55-65.
82. Schneider SH, Amorosa LF, Khachadurian AK, et al. Studies on the mechanism of improved glucose control during regular exercise in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1984;26:355-360.
83. Skarfors ET, Wegener TA, Lithell H, et al. Physical training as treatment for type 2 (non-insulin-dependent) diabetes in elderly men. A feasibility study over 2 years. Diabetologia. 1987;30:930-933.
84. Ouslander JG, Martin S. Assessment in the nursing home. Clin Geriatr Med. 1987;3:155-174.
85. Brock DB, Brody JA. Statistical and epidemiological characteristics. In: Andres R, Bierman EL, Hazzard WR, eds. Principles of Geriatric Medicine. New York; McGraw-Hill; 1985;3-71.
86. Kemper P, Murtaugh CM. Lifetime use of nursing home care. N Engl J Med. 1991;324:595-600.
87. Tonino RP. Diabetes education. What should health care providers in long-term nursing care facilities know about diabetes? Diabetes Care. 1990;13(suppl 2):55.
88. Harris MI, Hadden WC, Knowler WC, Bennett PH. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20-74 years. Diabetes. 1987;36:523-554.
89. Hing E, Sekscenski E, Strahan G. The national nursing home survey: 1985 summary for the United States. In: Vital and Health Statistics. DHHS-PHS 1989;89-1758. Washington, DC; Department of Health and Human Services; 1989.
90. Van Nostrand JF. Nursing home care for diabetics. In: Diabetes in America: Diabetes Data Compiled in 1984. DHHS pub 85-1468. Washington, DC: U.S. Government Printing Office; 1985.
91. Edwards WS, Winn DM, Kurlantzick V, Sheridan S, Berk ML, Retchin SC. Evaluation of national health interview survey diagnostic reporting. In: Vital and Health Statistics, Series 2, No. 120. DHHS 93-1394. Washington, DC; Department of Health and Human Services; 1994.
92. Mooradian AD, Osterweil D, Petrasek D, et al. Diabetes mellitus in elderly nursing home patients. A survey of clinical characteristics and management. J Am Geriatr Soc. 1988;36:391-396.
93. Lewis M, Kane RL, Cretin S, et al. The immediate and subsequent outcomes of nursing home care. Am J Public Health. 1985;75:758-762.
94. Tonino RP. What should health-care providers in long-term nursing care facilities know about diabetes. Diabetes Care. 1990;13:55-59.
95. Mooradian AD, Osterweil D, Petrasek D, Morley JE. Diabetes mellitus in elderly nursing home patients: a survey of clinical characteristics and management. J Am Geriatr Soc. 1988;16:391-396.
96. Fischer KF, Lees JA, Newman JH. Hypoglycemia in hospitalized patients. Cause and outcomes. N Engl J Med. 1986;315:1245-1250.
97. Aubert RE, Ballard DJ, Bennett PH, et al. Diabetes in America. In: National Institutes of Health: National Diabetes and Digestive and Kidney Diseases, 2nd Ed. NIH 95-1468. Washington, DC; National Institutes of Health; 1995.
98. Snyder NA, Feigal DW, Arieff AI. Hypernatremia in elderly patients. A heterogenous, morbid, and iatrogenic entity. Ann Intern Med. 1987;107:309-319.
99. Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46(4):701-710.
100. de Vegt F, Dekker JM, Jager A, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population. The Hoorn Study. JAMA. 2001;285(16):2109-2113.
101. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537-544.
102. Tuomilehto J, Lindstrom J, Eriksson JG, et at. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343-1350.
103. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393- 403.