Imaging fecal incontinence
Abstract
Fecal incontinence is the inability to defer release of gas or stool from the anus and rectum by mechanisms of voluntary control. It is an important medical disorder affecting the quality of life of up to 20% of the population above 65 years. The most common contributing factors include previous vaginal deliveries, pelvic or perineal trauma, previous anorectal surgery, and rectal prolapse. Many physicians lack experience and knowledge related to pelvic floor incontinence disorders, but advancing technology has improved this knowledge. Increased experience with endoanal ultrasound and endoanal magnetic resonance imaging have given us a better understanding not only of the anatomy of the anal canal but also of the underlying morphological defects in fecal incontinence. Current imaging methods are emphasized and recent literature is reviewed.
Author Keywords: Fecal incontinence; Defecography; Endoanal ultrasound; Magnetic resonance imaging; Endocoil
Fecal incontinence, the inability to deliberately control the anal sphincter, is a common disease and may affect up to 20% of the age group above 65 years. Fecal incontinence has a substantial impact on the quality of life. It is a socially disabling problem that prevents up to one-third of patients from seeking medical advice.
The most common causes include traumatic (obstetric, surgical) sphincter defects, neurogenic dysfunction of the musculature of the pelvic floor, and rectal prolapse. The prevalence of fecal incontinence in women is eight times higher than in men.
The most common cause of fecal incontinence in women is childbirth, where the sphincter muscles are commonly damaged. Traumatic rupture of the anal sphincters may result in immediate-onset fecal incontinence. Pudendal neuropathy, caused by stretching the branches of the pudendal nerve to the sphincter and levator ani as the fetal head pushes down on the pelvic floor to dilate the introitus, leads to delayed-onset incontinence. Following vaginal delivery, the pudendal nerve motor terminal latencies are increased for about 6 months and there is a fall in squeeze pressure regardless of sphincter damage. Nerve damage appears to be cumulative, whereas direct sphincter damage is most likely in the first delivery. An occult sphincter defect may precipitate overt symptoms later, as the effects of menopause, neuropathy, and muscle loss accumulate.
Diabetes may be associated with profound autonomic neuropathy, leading to dysfunction of the rectum and the sphincter muscles.
Abnormal thinning of the internal sphincter is common and of unknown etiology. Pelvic floor descent is common with advanced age. It may be secondary to prolonged straining, and the condition has been considered to cause primary damage to the pudendal nerves by stretching.
Indeed, in 80% of affected women, an obstetric trauma is present. Sultan et al. demonstrated that 35% of primiparous and 44% of multiparous women, after vaginal delivery, show defects of the internal and/or external sphincter muscles. One-third of these women also have direct disturbances of anal continence.
Fecal incontinence may be divided into passive, where leakage is the main problem, and urge, where stool cannot be held back. The passive form is more likely to be due to internal damage, while the urge incontinence can be attributed to external sphincter damage.
In treating fecal incontinence, the physician can choose from several modalities, depending on the localization of the impairment. Isolated external sphincter defects can be treated conservatively by physical therapy, including biofeedback. The pelvic floor may be trained to take on the function of the arbitrary sphincter muscle.
In isolated internal sphincter defects, physical therapy alone is unsuccessful because other muscles cannot compensate for the work of this involuntary muscle. Local measures and a dietetic or medicinally triggered increase of fecal consistency are necessary to obtain a good quality of life.
Patients with sphincter damage may benefit from surgical repair. To perform optimal surgery, an accurate description of the position, extent, and the type of the lesion is necessary. Postanal and sphincter repair are the established operative techniques. Long-term results of sphincter repair show success rates of 50–75%, although this technique repairs a circumscribed defect with intact surrounding muscle. Long-term results for postanal repair are not good due to primary diffuse impairment of sphincter function. Total pelvic repair has a success rate of 11%.
The choice of an optimal therapy is determined on the basis of a proper assessment, especially accurate images of the anal sphincter complex. Imaging methods applied are defecography, endoanal ultrasonography (US), and magnetic resonance (MR) imaging. Conventional defecography is important for the accurate diagnosis of intussusception and rectoceles. Endoanal ultrasound (EUS) is the preferred diagnostic technique and has replaced the invasive method of electromyography. Recently, MR imaging, especially with endorectal coils, has been shown to be accurate in delineating the anatomy of the sphincter complex.
2. Clinical diagnosis
The 4-grade classification after Browning and Parks has been the standard for clinically grading fecal incontinence for many years. Grade I means involuntary loss of gas, grade II is soiling, grade III means loss of solid material, and grade IV refers to complete incontinence. The Jorge and Wexner questionnaire, which comprises an estimation of leakage frequency with the need to wear a pad and the overall effect on lifestyle, has become widely used since its implemention in 1993. With this system, a score of 0 indicates perfect continence and a score of 20 indicates complete incontinence.
For the assessment of fecal incontinence, digital palpation, manometry (ballon- or vector-mamometry), and measurement of pudendal nerve motor latency are used. In manometry, pressure at rest and pressure at contraction are quantified in a station-pull-through technique. A low pressure at rest is due to functional impairment of the internal sphincter, and a low pressure at contraction is due to impairment of the external sphincter muscle. However, even modern vectormanometry cannot provide precise definition of localization and extent of sphincter defects. Pudendal nerve terminal latency is prolonged in patients with idiopathic fecal incontinence. Pudendal latency is increased in patients with long-standing constipation, perineal descent, or, generally, in pelvic floor disorders. However, with all of these methods, structural defects cannot be assessed .
3. Imaging methods
3.1. Defecography (=evacuation proctography)
Defecography documents the process of rectal evacuation. It provides objective information about rectocele size and emptying and demonstrates coexistent enteroceles. This radiographic technique is a useful method for the diagnosis of rectal intussusception, the mechanism by which rectal prolapse occurs.
The rectum is filled with up to 300 ml of a thick barium paste (to approximate the consistency of fecal material). The patient is seated on a radiolucent toilet chair mounted at the footplate of a remote-control stand. A series of lateral images and video films of the rectum and the anal canal are obtained during the process of rectal evacuation. Films are generally obtained at rest, on voluntary contraction of the anal sphincter and pelvic floor muscles, on straining down, and during defecation. The vagina is also opacified. To visualize the loops of the small bowel in the pelvis, barium contrast medium is given orally half an hour prior to examination.
A large number of incontinent patients have concomitant symptoms of pelvic outlet obstruction. In this group, defecography is useful for demonstrating large non-emptying rectoceles, a spastic pelvic floor, and intussusception.
Intussusception is the mechanism by which rectal prolapse occurs (Fig. 1). It usually starts as an infolding, 6–8 cm inside the rectum, originating from the anterior wall in an annular fashion. On continued straining - in patients with rectal prolapse - the intussusception advances down into the anal canal and then through the anal orifice, constituting a complete rectal prolapse.
 Fig. 1. An 80-year-old woman with fecal incontinence (soiling) and pelvic outlet obstruction. Ap-view of defecography shows circumferential intussusception (arrows).
Fig. 1. An 80-year-old woman with fecal incontinence (soiling) and pelvic outlet obstruction. Ap-view of defecography shows circumferential intussusception (arrows).
Rectoceles are evident at defecography as an outpouching of the anterior rectal wall during evacuation (Fig. 2). Enteroceles are suspected when a separation of the upper vagina from the adjacent rectum by 2 cm or more is present. The definite diagnosis, however, requires the demonstration of herniated loops of small bowel within the widened rectovaginal space (Fig. 3)
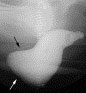 Fig. 2. A 57-year-old woman with fecal incontinence and pelvic outlet obstruction. Lateral view of defecography shows large anterior rectrocele with a diameter of 5 cm (arrows).
Fig. 2. A 57-year-old woman with fecal incontinence and pelvic outlet obstruction. Lateral view of defecography shows large anterior rectrocele with a diameter of 5 cm (arrows).
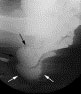 Fig. 3. A 60-year-old woman with pelvic outlet obstruction. Lateral view of defecography on straining shows large enterocele in rectovaginal space (black arrows) with compression of rectum (white arrows).
Fig. 3. A 60-year-old woman with pelvic outlet obstruction. Lateral view of defecography on straining shows large enterocele in rectovaginal space (black arrows) with compression of rectum (white arrows).
When the patient is seated for defecography, the weight of the abdominal contents stresses the pelvic floor to reveal the abnormal position of the pelvic floor at rest. Leakage of contrast at rest on defecography is also a good indication of sphincter weakness, and is found only in patients with overt fecal incontinence. This does depend on how much contrast is injected into the rectum. If the rectum is filled to the maximum tolerated capacity, the rectoanal reflex that causes leakage is invoked.
Defecography is also used to assess anismus and spastic pelvic floor syndrome. However, overall defecography is of rather limited value in incontinent patients unless they suffer from associated obstructive symptoms.
3.2. Endoanal ultrasound (EUS)
Since its implementation, EUS has been the gold standard in the morphological diagnosis of the anal canal. Muscular discontinuation of anal sphincters can be clearly discriminated of diffuse atony without structural defects. Moreover, EUS is easily available, cheap, and patient-compliant.
EUS is performed with dedicated equipment including a high-frequency mechanically rotated transducer for a 360° axial image. For acoustic coupling, the transducer is encased by a hard plastic cone, filled with degassed water. The plastic cone is covered with a condom after application of a lubricant to the surfaces of the condom and the cone. The probe is inserted into the rectum while the patient is in the left lateral or lithotomy position, and then rotated, so that the 12 o?clock position is anterior.
The reflectivity of muscle depends more on its fibroelastic content and the orientation of these fibers rather than on the type of muscle cells, as these on their own are all of low reflectivity. The internal sphincter has low fibroelastic content, and presents as a well-defined ring of low reflectivity. The external sphincter is more variable in fiber content.
With regard to the anal canal anatomy, three layers can be differentiated endosonographically: the highly reflective submucosa; the low-reflective internal sphincter muscle, about 2 mm thick; and the variably reflective external sphincter muscle. The deep part of the external anal sphincter merges with the puborectalis muscle dorsally, with the superficial ends at the caudal extent of the internal sphincter.
There is a difference between the sexes in the appearance of the external sphincter. In men it is more symmetric, less reflective, and easier to delineate, while in women the perineal body seems void of any structure, as it is mainly fibroelastic in content.
Any break in the continuity of the ring of low reflectiveness representing the internal sphincter muscle is abnormal and is considered to be indicative of direct trauma, particularly from surgical procedures such as an anal stretch, lateral internal anal sphincterotomy, fistulotomy for fistula, or as part of generalized sphincter trauma from vaginal delivery (Fig. 4). A general thinning may be found in internal sphincter degeneration.
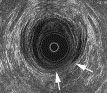 Fig. 4. A 45-year-old man with fecal incontinence (soiling) after fistulotomy. EUS image shows destruction of external sphincter muscle between 4 and 6 o’clock and internal sphincter between 3 and 6 o’clock (arrows).
Fig. 4. A 45-year-old man with fecal incontinence (soiling) after fistulotomy. EUS image shows destruction of external sphincter muscle between 4 and 6 o’clock and internal sphincter between 3 and 6 o’clock (arrows).
Tears in the external sphincter muscle are defined by an interruption of the fine parallel fibrillar echotexture. In childbirth, tears result from overstretching, or extension from an adjacent rupture or episiotomy (Fig. 5 and Fig. 6). Scars are characterized by loss of normal architecture with an area of amorphous texture that usually shows low reflectiveness corresponding to fibrosis. Generalized external sphincter atrophy is difficult to appreciate because of the vague contours of the muscle ring. Fat replacement within the atrophied muscle causes loss of the normal muscle/fat interface border at the outer margin of the external sphincter. The outer border of the external sphincter is then not defined, and thickness cannot be accurately measured. MR imaging is the more reliable method for evaluation in this instance.
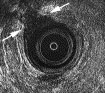 Fig. 5. A 56-year-old woman with fecal incontinence after episiotomy. EUS image shows amorphous zone of low reflectiveness between 10 and 12 o?clock (arrows) corresponding to circumscribed destruction of external sphincter muscle.
Fig. 5. A 56-year-old woman with fecal incontinence after episiotomy. EUS image shows amorphous zone of low reflectiveness between 10 and 12 o?clock (arrows) corresponding to circumscribed destruction of external sphincter muscle.
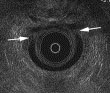 Fig. 6. A 63-year-old woman with fecal incontinence after three vaginal deliveries. EUS image shows large defect of internal and external anal sphincters between 10 and 2 o’clock (arrows).
Fig. 6. A 63-year-old woman with fecal incontinence after three vaginal deliveries. EUS image shows large defect of internal and external anal sphincters between 10 and 2 o’clock (arrows).
The main advantage of the use of EUS is the exact delineation of internal and external sphincter morphology with minimum discomfort for the patient. To date, EUS has almost completely replaced the painful needle-electromyographic mapping in use at the beginning of the last decade, and given a new comprehension of morphological changes of the sphincter muscles in the genesis of fecal incontinence. Operator dependence and common pitfalls, related to aberrant bundles of the external sphincter and gender-related anatomy, are currently the relative limitations of this method. EUS is more sensitive in the evaluation of fecal incontinence than anal manometry due to the morphologic depiction of sphincter defects. EUS should be mandatory in the assessment of fecal incontinence, even to confirm intact sphincter muscles in suspected neurogenic causes.
3.3. MR imaging
Endoanal MR imaging has the advantages of multiplanar imaging and high soft tissue contrast, especially for defining the components of the external sphincter muscle.
MR imaging is performed at 1.0 or 1.5 Tesla units and endoanal surface coils, with diameters ranging from 12 to 19 mm and lengths of 50–75 mm. The endoanal coil is covered with a condom and a lubricant, then applied with the patient rotated to the left. External coil holders can be used but may increase patient discomfort. The MR imaging examination is performed in the supine position after application of hyoscine butylbromide (Buscopan®) or Glucagon® IV to reduce peristaltic bowel movement.
An optimal imaging protocol for fecal incontinence has not been established to date. However, the use of T2-weighted turbo spin echo (TSE) and 3D-gradient echo sequences with a slice thickness of 2–4 mm in the axial, coronal, and sagittal orientation should be performed. The coronal and the sagittal planes should be oriented perpendicular to the long axis of the endoanal coil. The examination time should not exceed 30 min.
Recent literature has evaluated high-resolution MR imaging of the anal sphincter complex using phased-array coils, but its role in fecal incontinence must still be determined. For the visualization of sphincter defects, however, the use of an endoanal coil is necessary.
A sphincter defect is defined as a discontinuity of the muscle ring (Fig. 7). Scarring is defined as a hypointense deformation of the normal pattern of the muscle layer due to replacement of muscle cells by fibrous tissue.
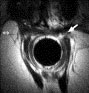 Fig. 7. A 53-year-old woman with fecal incontinence after obstetric trauma. Axial T2-weighted MR image of the anal canal using an endocoil shows discontinuity of the muscle ring at 2 o’clock, corresponding to a sphincter tear.
Fig. 7. A 53-year-old woman with fecal incontinence after obstetric trauma. Axial T2-weighted MR image of the anal canal using an endocoil shows discontinuity of the muscle ring at 2 o’clock, corresponding to a sphincter tear.
MR imaging is comparable to EUS with regard to the characterization of damage to the internal sphincter. With regard to characterization of damage to the external sphincter, however, MR imaging allows good distinction among muscles, scars, and fat tissue, which facilitates the accurate detection of local thinning, which is not possible with EUS, and gives a more precise description of the extent and structure of complex lesions.
Endocoil MR imaging allows an accurate assessment of the thickness and the fat content of the external sphincter. External sphincter atrophy is usually associated with generalized thinning and reduction of the striated muscle with fatty replacement (Fig. 8). Endosonography alone is not a reliable modality for this imaging task. MR imaging has been shown to be able to determine the presence or absence of external sphincter atrophy with a sensitivity of 89% and a specificity of 94%.
 Fig. 8. Coronal T2-weighted MR images of the anal canal with applied endorectal coil (large arrow). (A) A 38-year-old healthy volunteer. MR image shows normal anatomy with sphincter muscles of regular thickness (small arrows). (B) A 79-year-old woman with passive fecal incontinence. MR image shows generalized thinning and reduction of striated muscles with fatty replacement (arrows).
Fig. 8. Coronal T2-weighted MR images of the anal canal with applied endorectal coil (large arrow). (A) A 38-year-old healthy volunteer. MR image shows normal anatomy with sphincter muscles of regular thickness (small arrows). (B) A 79-year-old woman with passive fecal incontinence. MR image shows generalized thinning and reduction of striated muscles with fatty replacement (arrows).
Recently, dynamic MR defecography, with the advantages of a dynamic analysis of pelvic floor disorders in anorectal disease, has been introduced.
4. Conclusion
Fecal incontinence is often multifactoral. Clinical examination may not detect the cause in at least 25% of patients.
Defecography provides both structural and functional information for rectal voiding and prolapse. Defecography may demonstrate pelvic floor and sphincter weakness by abnormal descent at rest and anal leakage.
EUS and MR imaging are complementary with regard to surgical decision-making. The advantages of EUS are, as stated above, that it is cheaper, more widely available, and a quicker technique than MR imaging.
EUS is valuable as a screening procedure to detect sphincter tears amenable to surgical treatment. EUS is the method of choice to show damage to the sphincter muscles, either with general thinning as found in degeneration, or from focal discontinuities due to any cause of trauma. MR imaging is a powerful tool with which to investigate weakness of the pelvic floor generally and atrophy of the external sphincter, an important predictive factor for the outcome of sphincter repair.
References
1. R.O. Roberts, S.J. Jacobson, W.T. Reilly, J.H. Pemberton, M.M. Lieber and N.J. Talley, Prevalence of combined fecal and urinary incontinence: a community-based study. J. Am. Geriatr. Soc. 47 (1999), pp. 837–841.
2. M.R.B. Keighley and N.S. Williams, Faecal incontinence. In: M.R.B. Keighley and N.S. Williams, Editors, Surgery of The Anus, Rectum and Colon, Saunders, London (1993), pp. 592–700.
3. A.H. Sultan, M.A. Kamm, C.I. Bartram and C.N. Hudson, Anal sphincter trauma during instrumental delivery. Int. J. Gynecol. Obstet. 43 (1993), pp. 263–270.
4. A.H. Sultan, M.A. Kamm, C.N. Hudson, J.M. Thomas and C.I. Bartram, Anal-sphincter disruption during vaginal delivery. N. Engl. J. Med. 329 (1993), pp. 1905–1911.
5. A.H. Sultan, M.A. Kamm, R.J. Nicholls and C.I. Bartram, Prospective study of the extent of sphincter division during lateral sphincterotomy. Dis. Colon Rectum 37 (1994), pp. 1031–1033.
6. C.I. Bartram, Faecal incontinence. In: C.I. Bartram and J.O.L. DeLancey, Editors, Imaging Pelvic Floor Disorders, Springer, Berlin (2003), pp. 159–164.
7. A.G. Parks and J.D. Hardcastle, The syndrome of the descending perineum. Proc. R. Soc. Med. 59 (1966), pp. 477–482.
8. R.J.F. Felt-Bersma, Miguel Cuesta and Martine Koorevaar, Anal sphincter repair improves anorectal function and endosonographic image: a prospective clinical study. Dis. Colon Rectum 39 (1996), pp. 878–885.
9. P. Setti Carraro, M.A. Kamm and R.J. Nicholls, Long-term results of postanal repair for neurogenic faecal incontinence. Br. J. Surg. 81 (1994), pp. 140–144.
10. M. Pinho, J. Ortiz, M. Oya, B. Panagamuwa, J. Asperer and M.R. Keighley, Total pelvic floor repair for the treatment of neuropathic faecal incontinence. Am. J. Surg. 163 (1992), pp. 340–343.
11. G.G. Browning and A.G. Parks, Postanal repair for neuropathic faecal incontinence: correlation of clinical results and anal canal pressures. Br. J. Surg. 70 2 (1983), pp. 101–104.
12. J.M. Jorge and S.D. Wexner, Etiology and management of fecal incontinence. Dis. Colon Rectum 36 (1993), pp. 77–97.
Michael H. Fuchsjäger and Andrea G. Maier
Department of Radiology, University of Vienna, Währinger Gürtel 18-20, A-1090, Vienna, Austria
Received 19 May 2003; revised 20 May 2003; accepted 21 May 2003. ; Available online 18 July 2003.